|
Synonyms: Hengqu® | SHR-8735
hetrombopag is an approved drug (China (2021))
Compound class:
Synthetic organic
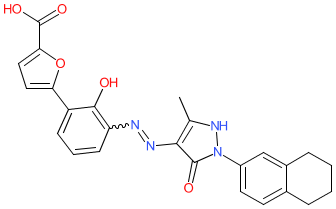
Comment: Hetrombopag is an orally bioavailable, non-peptide small‐molecule thrombopoietin receptor (TPOR/MPL) agonist, that was developed as a treatment for thrombocytopenia [1]. The parent compound is shown here. It is administered as the ethanolamine/olamine.
|
|
|||||||||||||||||||||||||||||||||||
| No information available. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02403440 | A Study of Hetrombopag Olamine in Chronic Idiopathic Thrombocytopenic Purpura (ITP) Patients | Phase 1 Interventional | Jiangsu HengRui Medicine Co., Ltd. | ||
| NCT03222843 | Multicentre, Randomised Phase III Study of the Efficacy and Safety of Hetrombopag Olamine in Idiopathic Thrombocytopenic Purpura (ITP) Patient | Phase 3 Interventional | Jiangsu HengRui Medicine Co., Ltd. | ||
| NCT03603132 | A Study to Evaluate Different Intervals Between Dosing and Feeding on the Pharmacokinetics | Phase 1 Interventional | Jiangsu HengRui Medicine Co., Ltd. | Results from this study show that hetrombopag is most effective (in terms of elevating platelet numbers) when administered on an empty stomach (fasting condition) or at least 2 hours before a meal. | 2 |