|
Synonyms: Mulpleta® | S-888711 | S888711
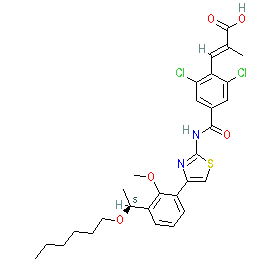
lusutrombopag is an approved drug (Japan (2015), FDA (2018), EMA (2019))
Compound class:
Synthetic organic
Comment: Lusutrombopag is an orally bioavailable, nonpeptidyl, small molecule thrombopoietin (TPO) receptor agonist [4] that was developed by Shionogi in Japan. It was the second TPO receptor agonist to receive FDA approval in 2018 (avatrombopag was the first).
Lusutrombopag demonstrates antibacterial activity in vitro with potential to be repurposed as a treatment for infections caused by drug-resistant Enterococcus spp. [3]. |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| Lusutrombopag is selective for the human TPO receptor, and does not activate the mouse TPO receptor [4]. It promotes proliferation of Ba/F3-hMpl cells with an EC50 of 84 nM via activation of the same signal transduction pathways as native human TPO. Incubation of HuBM-CD34-positive cells with lusutrombopag for 12 days in vitro promotes megakaryocytic colony formation, which is indicative of enhanced platelet formation. Lusutrombopag has in vitro antibacterial activity against vancomycin-resistant strains of Enterococcus faecium and Enterococcus faecalis (MIC of 2-4 μg/ml) and demonstrates a synergistic effect in combination with aminoglycoside antibacterial drugs [3]. |
| Selectivity at catalytic receptors | ||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||