Contents:
- Gene and Protein Information
- Previous and Unofficial Names
- Database Links
- Selected 3D Structures
- Associated Proteins
- Natural/Endogenous Ligands
- Agonists
- Transduction Mechanisms
- Tissue Distribution
- Expression Datasets
- Functional Assays
- Physiological Consequences of Altering Gene Expression
- Xenobiotics Influencing Gene Expression
- Phenotypes, Alleles and Disease Models
- Clinically-Relevant Mutations and Pathophysiology
- General Comments
- References
- Contributors
- How to cite this page
Gene and Protein Information  |
||||||
| Adhesion G protein-coupled receptor | ||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 7 | 1474 | 19p13.12 | ADGRL1 | adhesion G protein-coupled receptor L1 | |
| Mouse | 7 | 1466 | 8 C2 | Adgrl1 | adhesion G protein-coupled receptor L1 | |
| Rat | 7 | 1515 | 19q11 | Adgrl1 | adhesion G protein-coupled receptor L1 | |
Previous and Unofficial Names  |
| LPHN1 (latrophilin 1) | LEC1 (lectomedin-2) | CIRL1 (calcium-independent alpha-latrotoxin 1) | CL1 (CIRL/latrophilin 1) |
Database Links  |
|
| Specialist databases | |
| GPCRdb | lphn1_human (Hs), lphn1_mouse (Mm), agrl1_rat (Rn) |
| Other databases | |
| Alphafold | O94910 (Hs), Q80TR1 (Mm), O88917 (Rn) |
| CATH/Gene3D | 2.130.10.10 |
| Ensembl Gene | ENSG00000072071 (Hs), ENSMUSG00000013033 (Mm), ENSRNOG00000029134 (Rn) |
| Entrez Gene | 22859 (Hs), 330814 (Mm), 65096 (Rn) |
| Human Protein Atlas | ENSG00000072071 (Hs) |
| KEGG Gene | hsa:22859 (Hs), mmu:330814 (Mm), rno:65096 (Rn) |
| Pharos | O94910 (Hs) |
| RefSeq Nucleotide | NM_001008701 (Hs), NM_014921 (Hs), NM_181039 (Mm), NM_022962 (Rn) |
| RefSeq Protein | NP_055736 (Hs), NP_001008701 (Hs), NP_851382 (Mm), NP_075251 (Rn) |
| UniProtKB | O94910 (Hs), Q80TR1 (Mm), O88917 (Rn) |
| Wikipedia | ADGRL1 (Hs) |
Selected 3D Structures  |
|||||||||||
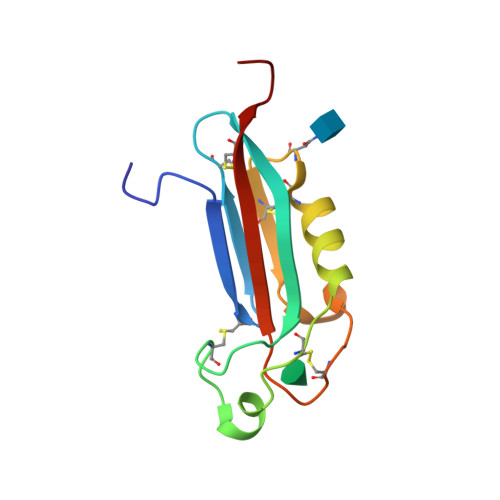
|
|
||||||||||
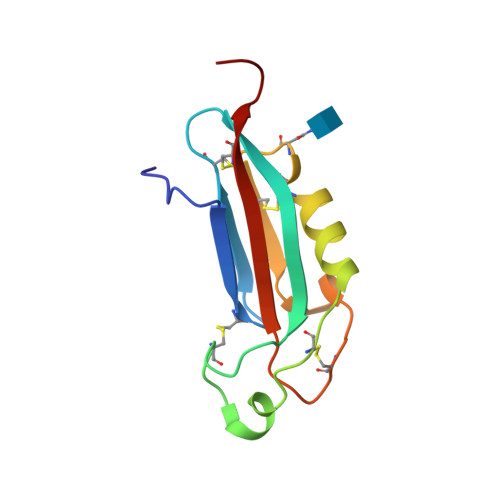
|
|
||||||||||
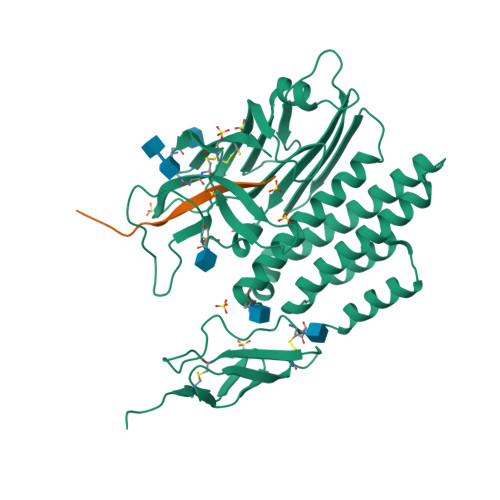
|
|
||||||||||
| Associated Protein Comments | ||
| Extracellular and transmembrane interactors: teneurin-2 [25], FLRT-1, -3 [19], neurexin-α1, -β1, -β2, -β3 [5], contactin-6 [31]. Intracellular interactors: shank [13], TRIP8b [20-21]. |
Natural/Endogenous Ligands  |
| lasso D |
Download all structure-activity data for this target as a CSV file 
| Agonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Agonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Neurexins 1β, 2β and 1α have been reported to bind ADGRL1 but have not been shown to stimulate it or cause any effect, except limited increase in adhesion between ADGRL1-transfected and NRXN-transfected HEK293 cells [5]. O'Sullivan et al. suggest in their 2012 publication that ADGRL1 might interact with FLRT3; however, the paper mainly deals with ADGRL3 and shows only indirect data on ADGRL1/FLRT3 interaction and does not report any physiological effect of any such interaction [19]. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Primary Transduction Mechanisms 
|
|
| Transducer | Effector/Response |
|
Gs family Gi/Go family Gq/G11 family |
Phospholipase C stimulation Potassium channel Calcium channel |
| References: 10,14-15,18,22,24-25,30 | |
Secondary Transduction Mechanisms  |
|
| Transducer | Effector/Response |
| G protein (identity unknown) | Adenylyl cyclase stimulation |
| Comments: The involvement of G proteins has not been shown in this case, although ADGRL1 has been demonstrated to bind G0 and Gq, but not Gi [15,22]. | |
| References: 15 | |
Tissue Distribution 
|
||||||||
|
||||||||
|
||||||||
|
Expression Datasets  |
|
|
Functional Assays 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Physiological Consequences of Altering Gene Expression 
|
||||||||||
|
Xenobiotics Influencing Gene Expression 
|
||||||||||
|
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| General Comments |
|
ADGRL1 (adhesion G protein-coupled receptor L1, formerly LPHN1 or latrophilin 1) is a receptor that belongs to Family I Adhesion-GPCRs along with ADGRL2 (latrophilin 2), ADGRL3 (latrophilin 3) and ADGRL4 (ELTD1) [3]. Family I Adhesion-GPCRs have orthologs in vertebrate and invertebrate species. There are clear problems with the distribution of ADGRL1 as determined by different techniques. Multiple Northern and Western blotting experiments [9,15,17,25-26] demonstrate that ADGRL1 mRNA and protein are distributed very similarly in rat and human tissues: ADGRL1 is almost exclusively expressed in the brain, is barely detectable in kidneys, lung and pancreas, and is undetectable in the liver. However, the two qPCR datasets [8,23] contradict both these data and each other. According to [8] ADGRL1 is ubiquitous in the rat and is highly abundant in rat liver, which is inconsistent with the blotting data. On the other hand, the same dataset reports that ADGRL1 is brain-specific in the mouse and is undetectable in mouse liver. While consistent with blotting, this clearly contradicts the second qPCR dataset [23], which shows that ADGRL1 is ubiquitous in the mouse and is highly expressed in mouse liver. One possible reason for this conflict could be a varying degree of contamination of tissue samples with autonomic neurons and endocrine cells, which both highly express ADGRL1. Like most other Adhesion-GPCRs, ADGRL1 is cleaved post-translationally into two subunits: α (also called N-terminal fragment, NTF) and β (also called C-terminal fragment, CTF) [11,30]. These subunits can behave as separate proteins on the cell surface; their re-association is driven by exogenous agonist binding and leads to intracellular calcium signalling [24,30]. This feature is not completely understood but is crucial for the functions of ADGRL1 and many other Adhesion GPCRs. There are multiple splice variants of ADGRL1 in all organisms, and the rat variant currently listed on the IUPHAR website (1515 amino acids) is less similar to the human ADGRL1 and less common than the two most abundant splice variants of rat ADGRL1 (1469 aa, accession AAC98700, [15]; and 1471 aa, accession NP_075251, [12]). One phenotype was in fact reported in the original paper on LPHN1-KO [27]: 'abnormal maternal nurturing' (Phenotype ID MP 0001386). Other phenotypes, like 'abnormal and decreased synaptic glutamate release' (MP 0004494 and MP 0004495) were seen only in response to toxin (α-latrotoxin) application and were not observed under physiological conditions. The Gal_lectin domain is now termed rhamnose-binding lectin (RBL) domain. |
References
1. Araç D, Boucard AA, Bolliger MF, Nguyen J, Soltis SM, Südhof TC, Brunger AT. (2012) A novel evolutionarily conserved domain of cell-adhesion GPCRs mediates autoproteolysis. EMBO J, 31 (6): 1364-78. [PMID:22333914]
2. Ashton AC, Volynski KE, Lelianova VG, Orlova EV, Van Renterghem C, Canepari M, Seagar M, Ushkaryov YA. (2001) alpha-Latrotoxin, acting via two Ca2+-dependent pathways, triggers exocytosis of two pools of synaptic vesicles. J Biol Chem, 276 (48): 44695-703. [PMID:11572875]
3. Bjarnadóttir TK, Fredriksson R, Höglund PJ, Gloriam DE, Lagerström MC, Schiöth HB. (2004) The human and mouse repertoire of the adhesion family of G-protein-coupled receptors. Genomics, 84 (1): 23-33. [PMID:15203201]
4. Bonaglia MC, Marelli S, Novara F, Commodaro S, Borgatti R, Minardo G, Memo L, Mangold E, Beri S, Zucca C et al.. (2010) Genotype-phenotype relationship in three cases with overlapping 19p13.12 microdeletions. Eur J Hum Genet, 18 (12): 1302-9. [PMID:20648052]
5. Boucard AA, Ko J, Südhof TC. (2012) High affinity neurexin binding to cell adhesion G-protein-coupled receptor CIRL1/latrophilin-1 produces an intercellular adhesion complex. J Biol Chem, 287 (12): 9399-413. [PMID:22262843]
6. Chen ML, Chen CH. (2005) Microarray analysis of differentially expressed genes in rat frontal cortex under chronic risperidone treatment. Neuropsychopharmacology, 30 (2): 268-77. [PMID:15536490]
7. Deák F, Liu X, Khvotchev M, Li G, Kavalali ET, Sugita S, Südhof TC. (2009) Alpha-latrotoxin stimulates a novel pathway of Ca2+-dependent synaptic exocytosis independent of the classical synaptic fusion machinery. J Neurosci, 29 (27): 8639-48. [PMID:19587270]
8. Haitina T, Olsson F, Stephansson O, Alsiö J, Roman E, Ebendal T, Schiöth HB, Fredriksson R. (2008) Expression profile of the entire family of Adhesion G protein-coupled receptors in mouse and rat. BMC Neurosci, 9: 43. [PMID:18445277]
9. Ichtchenko K, Bittner MA, Krasnoperov V, Little AR, Chepurny O, Holz RW, Petrenko AG. (1999) A novel ubiquitously expressed alpha-latrotoxin receptor is a member of the CIRL family of G-protein-coupled receptors. J Biol Chem, 274 (9): 5491-8. [PMID:10026162]
10. Ichtchenko K, Khvotchev M, Kiyatkin N, Simpson L, Sugita S, Südhof TC. (1998) alpha-latrotoxin action probed with recombinant toxin: receptors recruit alpha-latrotoxin but do not transduce an exocytotic signal. EMBO J, 17 (21): 6188-99. [PMID:9799228]
11. Krasnoperov V, Lu Y, Buryanovsky L, Neubert TA, Ichtchenko K, Petrenko AG. (2002) Post-translational proteolytic processing of the calcium-independent receptor of alpha-latrotoxin (CIRL), a natural chimera of the cell adhesion protein and the G protein-coupled receptor. Role of the G protein-coupled receptor proteolysis site (GPS) motif. J Biol Chem, 277 (48): 46518-26. [PMID:12270923]
12. Krasnoperov VG, Bittner MA, Beavis R, Kuang Y, Salnikow KV, Chepurny OG, Little AR, Plotnikov AN, Wu D, Holz RW et al.. (1997) alpha-Latrotoxin stimulates exocytosis by the interaction with a neuronal G-protein-coupled receptor. Neuron, 18 (6): 925-37. [PMID:9208860]
13. Kreienkamp HJ, Zitzer H, Gundelfinger ED, Richter D, Bockers TM. (2000) The calcium-independent receptor for alpha-latrotoxin from human and rodent brains interacts with members of the ProSAP/SSTRIP/Shank family of multidomain proteins. J Biol Chem, 275 (42): 32387-90. [PMID:10964907]
14. Lajus S, Vacher P, Huber D, Dubois M, Benassy MN, Ushkaryov Y, Lang J. (2006) Alpha-latrotoxin induces exocytosis by inhibition of voltage-dependent K+ channels and by stimulation of L-type Ca2+ channels via latrophilin in beta-cells. J Biol Chem, 281 (9): 5522-31. [PMID:16301314]
15. Lelianova VG, Davletov BA, Sterling A, Rahman MA, Grishin EV, Totty NF, Ushkaryov YA. (1997) Alpha-latrotoxin receptor, latrophilin, is a novel member of the secretin family of G protein-coupled receptors. J Biol Chem, 272 (34): 21504-8. [PMID:9261169]
16. Lelyanova VG, Thomson D, Ribchester RR, Tonevitsky EA, Ushkaryov YA. (2009) Activation of alpha-latrotoxin receptors in neuromuscular synapses leads to a prolonged splash acetylcholine release. Bull Exp Biol Med, 147 (6): 701-3. [PMID:19902061]
17. Matsushita H, Lelianova VG, Ushkaryov YA. (1999) The latrophilin family: multiply spliced G protein-coupled receptors with differential tissue distribution. FEBS Lett, 443 (3): 348-52. [PMID:10025961]
18. Müller A, Winkler J, Fiedler F, Sastradihardja T, Binder C, Schnabel R, Kungel J, Rothemund S, Hennig C, Schöneberg T et al.. (2015) Oriented Cell Division in the C. elegans Embryo Is Coordinated by G-Protein Signaling Dependent on the Adhesion GPCR LAT-1. PLoS Genet, 11 (10): e1005624. [PMID:26505631]
19. O'Sullivan ML, de Wit J, Savas JN, Comoletti D, Otto-Hitt S, Yates 3rd JR, Ghosh A. (2012) FLRT proteins are endogenous latrophilin ligands and regulate excitatory synapse development. Neuron, 73 (5): 903-10. [PMID:22405201]
20. Popova NV, Plotnikov A, Deev IE, Petrenko AG. (2007) Interaction of calcium-independent latrotoxin receptor with intracellular adapter protein TRIP8b. Dokl Biochem Biophys, 414: 149-51. [PMID:17695324]
21. Popova NV, Plotnikov AN, Ziganshin RKh, Deyev IE, Petrenko AG. (2008) Analysis of proteins interacting with TRIP8b adapter. Biochemistry Mosc, 73 (6): 644-51. [PMID:18620529]
22. Rahman MA, Ashton AC, Meunier FA, Davletov BA, Dolly JO, Ushkaryov YA. (1999) Norepinephrine exocytosis stimulated by alpha-latrotoxin requires both external and stored Ca2+ and is mediated by latrophilin, G proteins and phospholipase C. Philos Trans R Soc Lond, B, Biol Sci, 354 (1381): 379-86. [PMID:10212487]
23. Regard JB, Sato IT, Coughlin SR. (2008) Anatomical profiling of G protein-coupled receptor expression. Cell, 135 (3): 561-71. [PMID:18984166]
24. Silva JP, Lelianova V, Hopkins C, Volynski KE, Ushkaryov Y. (2009) Functional cross-interaction of the fragments produced by the cleavage of distinct adhesion G-protein-coupled receptors. J Biol Chem, 284 (10): 6495-506. [PMID:19124473]
25. Silva JP, Lelianova VG, Ermolyuk YS, Vysokov N, Hitchen PG, Berninghausen O, Rahman MA, Zangrandi A, Fidalgo S, Tonevitsky AG et al.. (2011) Latrophilin 1 and its endogenous ligand Lasso/teneurin-2 form a high-affinity transsynaptic receptor pair with signaling capabilities. Proc Natl Acad Sci USA, 108 (29): 12113-8. [PMID:21724987]
26. Sugita S, Ichtchenko K, Khvotchev M, Südhof TC. (1998) alpha-Latrotoxin receptor CIRL/latrophilin 1 (CL1) defines an unusual family of ubiquitous G-protein-linked receptors. G-protein coupling not required for triggering exocytosis. J Biol Chem, 273 (49): 32715-24. [PMID:9830014]
27. Tobaben S, Südhof TC, Stahl B. (2002) Genetic analysis of alpha-latrotoxin receptors reveals functional interdependence of CIRL/latrophilin 1 and neurexin 1 alpha. J Biol Chem, 277 (8): 6359-65. [PMID:11741895]
28. Vakonakis I, Langenhan T, Prömel S, Russ A, Campbell ID. (2008) Solution structure and sugar-binding mechanism of mouse latrophilin-1 RBL: a 7TM receptor-attached lectin-like domain. Structure, 16 (6): 944-53. [PMID:18547526]
29. Volynski KE, Capogna M, Ashton AC, Thomson D, Orlova EV, Manser CF, Ribchester RR, Ushkaryov YA. (2003) Mutant alpha-latrotoxin (LTXN4C) does not form pores and causes secretion by receptor stimulation: this action does not require neurexins. J Biol Chem, 278 (33): 31058-66. [PMID:12782639]
30. Volynski KE, Silva JP, Lelianova VG, Atiqur Rahman M, Hopkins C, Ushkaryov YA. (2004) Latrophilin fragments behave as independent proteins that associate and signal on binding of LTX(N4C). EMBO J, 23 (22): 4423-33. [PMID:15483624]
31. Zuko A, Oguro-Ando A, Post H, Taggenbrock RL, van Dijk RE, Altelaar AF, Heck AJ, Petrenko AG, van der Zwaag B, Shimoda Y et al.. (2016) Association of Cell Adhesion Molecules Contactin-6 and Latrophilin-1 Regulates Neuronal Apoptosis. Front Mol Neurosci, 9: 143. [PMID:28018171]










