Contents:
- Gene and Protein Information
- Previous and Unofficial Names
- Database Links
- Selected 3D Structures
- Natural/Endogenous Ligands
- Agonists
- Antagonists
- Immunopharmacology Comments
- Immuno Cell Type Associations
- Immuno Process Associations
- Transduction Mechanisms
- Tissue Distribution
- Expression Datasets
- Functional Assays
- Physiological Functions
- Physiological Consequences of Altering Gene Expression
- Phenotypes, Alleles and Disease Models
- Clinically-Relevant Mutations and Pathophysiology
- General Comments
- References
- Contributors
- How to cite this page
Gene and Protein Information  |
||||||
| class A G protein-coupled receptor | ||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 7 | 412 | 22q11.23 | ADORA2A | adenosine A2a receptor | 43,72,108 |
| Mouse | 7 | 410 | 10 C1 | Adora2a | adenosine A2a receptor | 85 |
| Rat | 7 | 410 | 20p12 | Adora2a | adenosine A2a receptor | 22 |
Previous and Unofficial Names  |
| RDC8 | A2-AR | adenosine receptor A2a |
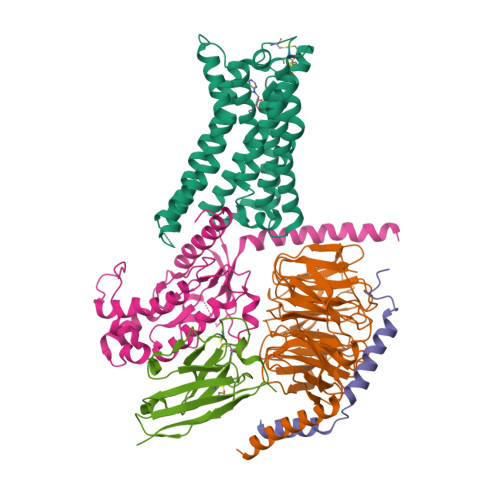
Selected 3D Structures  |
|||||||||||||
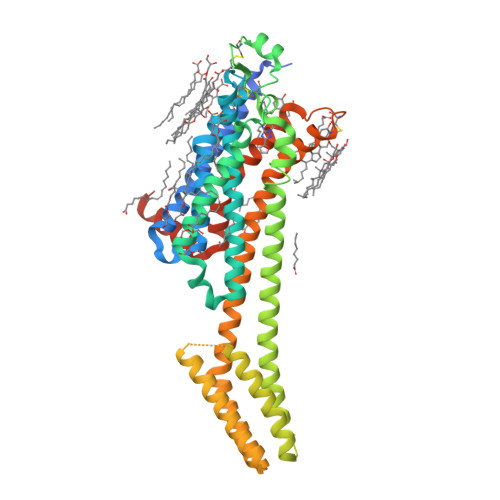
|
|
||||||||||||
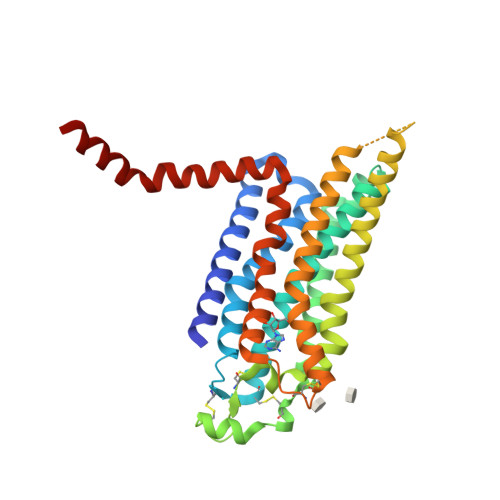
|
|
||||||||||||
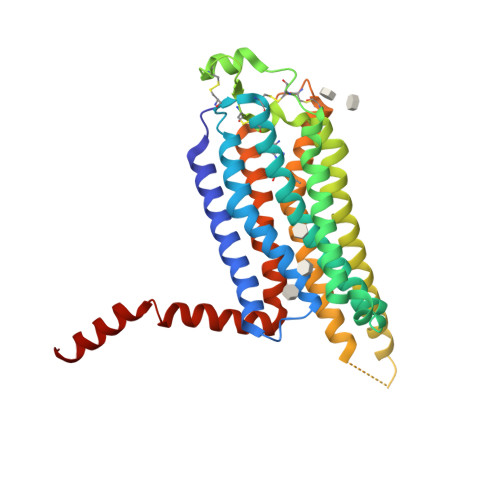
|
|
||||||||||||
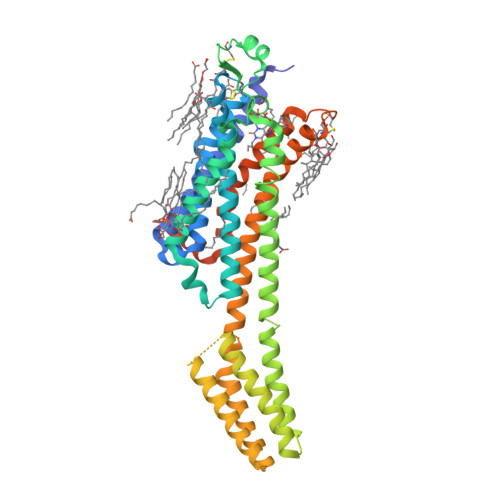
|
|
||||||||||||
|
|
||||||||||||
Natural/Endogenous Ligands  |
| adenosine |
Download all structure-activity data for this target as a CSV file 
| Agonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific agonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Agonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| The INN-assigned compound evodenoson is an agonist of the A2A receptor but affinity data is not available. Note that for tecadenoson, there is inconsistency between the two referenced articles (from the same group) as to the species origin of the ADORA2A used to generate the Ki value which is presented as identical in both articles. We have used the earlier paper as precedent, which indicates the use of the porcine receptor. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonists | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific antagonist tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Antagonist Comments | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Some istradefylline pKi values are derived from unpublished data (Müller et al.). The human MSX-2 pKi values are derived either from experiments using recombinant receptors expressed in CHO (the higher pKi) or from native receptors in human brain tissue (the lower pKi). |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immunopharmacology Comments |
| Agonist stimulation of the A2A and A3 receptors down-regulates production of the pro-inflammatory mediators TNF-α and IL-8 in human synoviocytes [139], suggesting a role in controlling arthritic joint inflammation. Experimental evidence indicates that A2A receptor-mediated mechanisms regulate the cytokine secretion pattern of iNKT cells [100]. Arizmendi and Kulka (2018) have demonstrated that adenosine, acting via the A2A receptor, inhibits C3a-mediated activation of human mast cells through a Gαs-dependent pathway, leading the authors to conclude that this pathway may be an important regulator of mast cell activation that is a tractable target for pharmacological intervention in mast cell-mediated allergic inflammation [6]. The A2A and A2B receptors on immune cells (e.g. T cells, NK cells, dendritic cells and macrophages) mediate the immunosuppressive effects of adenosine, an action that produces a powerful tolerogenic/immunosuppressed state in the tumour microenvironment. As a result, the A2A receptor has been suggested as an immune checkpoint that produces a negative feedback loop in the tumour microenvironment, that is hypothesised to be susceptible to A2A antagonist circumvention as a novel immuno-oncology approach [76]. This could potentially repurpose A2A receptor antagonists that have failed late stage clinical trials (Phase 3) as Parkinson's disease (PD) modulators, such as istradefylline (approved in Japan, but failed to gain US FDA approval for PD), preladenant, ST-1535 and tozadenant, amongst others. A2A receptor is discussed in this immuno-oncology review [2]. The potential therapeutic combination of A2A receptor antagonists with other checkpoint inhibitors in oncology is reveiwed by Garber (2017) [46], and Table 1 therein highlights some of the A2A antagonist/checkpoint inhibitor combinations that have synergistic anti-cancer potential. Examples of A2A antagonists in early stage clinical trials in advanced malignancies: AstraZeneca's AZD4635 (compound 4g [PMID: 22220592]) in combination with anti-PD-L1 durvalumab (NCT02740985), Merck's preladenant in combination with anti-PD-L1 pembrolizumab (NCT03099161), Palobiofarma's PBF-509 ([87]) in combination with anti-PD-1 investigational mAb spartalizumab (PDR001, NCT02403193), and Corvus Pharma/Genentech's ciforadenant (CPI-444) in combination with anti-PD-L1 atezolizumab (NCT02655822). Arcus Biosciences' AB928 (structure not disclosed) is a dual A2A/2B antagonist that is in early stage immuno-oncology development [125]. AB928 activity is suggested to be peripherally restricted and is devoid of the CNS-mediated pharmacology of other adenosine receptor antagonists. |
| Cell Type Associations | ||||||||
|
||||||||
|
| Immuno Process Associations | ||
|
||
|
||
|
||
|
||
|
Primary Transduction Mechanisms 
|
|
| Transducer | Effector/Response |
| Gs family | Adenylyl cyclase stimulation |
| References: 103 | |
Secondary Transduction Mechanisms  |
|
| Transducer | Effector/Response |
| Gq/G11 family |
Adenylyl cyclase stimulation Phospholipase C stimulation |
| Comments: A2A receptors have also been shown to couple to adenylate cyclase via Golf in the striatum [69] | |
| References: 42 | |
Tissue Distribution 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
Expression Datasets  |
|
|
Functional Assays 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Physiological Functions 
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
||||||||
|
Physiological Consequences of Altering Gene Expression 
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
||||||||||
|
Phenotypes, Alleles and Disease Models 
|
Mouse data from MGI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||
|
||||||||||
| General Comments |
| A2A antagonists can be useful as therapy for Parkinson's disease (see reviews [17,57,111]). Istradefylline (see [58] for a review) is marketed in Japan and the USA for this indication. |
References
1. Abo-Salem OM, Hayallah AM, Bilkei-Gorzo A, Filipek B, Zimmer A, Müller CE. (2004) Antinociceptive effects of novel A2B adenosine receptor antagonists. J Pharmacol Exp Ther, 308 (1): 358-66. [PMID:14563788]
2. Adams JL, Smothers J, Srinivasan R, Hoos A. (2015) Big opportunities for small molecules in immuno-oncology. Nat Rev Drug Discov, 14 (9): 603-22. [PMID:26228631]
3. Adén U, Halldner L, Lagercrantz H, Dalmau I, Ledent C, Fredholm BB. (2003) Aggravated brain damage after hypoxic ischemia in immature adenosine A2A knockout mice. Stroke, 34 (3): 739-44. [PMID:12624301]
4. Alexander SP, Millns PJ. (2001) [(3)H]ZM241385--an antagonist radioligand for adenosine A(2A) receptors in rat brain. Eur J Pharmacol, 411 (3): 205-10. [PMID:11164377]
5. Ali RA, Gandhi AA, Meng H, Yalavarthi S, Vreede AP, Estes SK, Palmer OR, Bockenstedt PL, Pinsky DJ, Greve JM et al.. (2019) Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat Commun, 10 (1): 1916. [PMID:31015489]
6. Arizmendi N, Kulka M. (2018) Adenosine activates Gαs proteins and inhibits C3a-induced activation of human mast cells. Biochem Pharmacol, 156: 157-167. [PMID:30099007]
7. Baraldi PG, Tabrizi MA, Preti D, Bovero A, Romagnoli R, Fruttarolo F, Zaid NA, Moorman AR, Varani K, Gessi S et al.. (2004) Design, synthesis, and biological evaluation of new 8-heterocyclic xanthine derivatives as highly potent and selective human A2B adenosine receptor antagonists. J Med Chem, 47 (6): 1434-47. [PMID:14998332]
8. Beatty J, Debien L, Jeffery J, Leleti MR, Mandal D, Miles D, Powers J, Rosen B, Thomas_tran R, Sharif E. (2018) AZOLOPYRIMIDINE FOR THE TREATMENT OF CANCER-RELATED DISORDERS. Patent number: WO2018136700. Assignee: ARCUS BIOSCIENCES, INC. Priority date: 19/01/2018. Publication date: 26/07/2018.
9. Betti M, Catarzi D, Varano F, Falsini M, Varani K, Vincenzi F, Pasquini S, di Cesare Mannelli L, Ghelardini C, Lucarini E et al.. (2019) Modifications on the Amino-3,5-dicyanopyridine Core To Obtain Multifaceted Adenosine Receptor Ligands with Antineuropathic Activity. J Med Chem, 62 (15): 6894-6912. [PMID:31306001]
10. Beukers MW, Wanner MJ, Von Frijtag Drabbe Künzel JK, Klaasse EC, IJzerman AP, Koomen GJ. (2003) N6-cyclopentyl-2-(3-phenylaminocarbonyltriazene-1-yl)adenosine (TCPA), a very selective agonist with high affinity for the human adenosine A1 receptor. J Med Chem, 46 (8): 1492-503. [PMID:12672250]
11. Bonizzoni E, Milani S, Ongini E, Casati C, Monopoli A. (1995) Modeling hemodynamic profiles by telemetry in the rat. A study with A1 and A2a adenosine agonists. Hypertension, 25 (4 Pt 1): 564-9. [PMID:7721399]
12. Borodovsky A, Barbon CM, Wang Y, Ye M, Prickett L, Chandra D, Shaw J, Deng N, Sachsenmeier K, Clarke JD et al.. (2020) Small molecule AZD4635 inhibitor of A 2A R signaling rescues immune cell function including CD103 + dendritic cells enhancing anti-tumor immunity. J Immunother Cancer, 8 (2): e000417. DOI: 10.1136/jitc-2019-000417 [PMID:32727810]
13. Borrmann T, Hinz S, Bertarelli DC, Li W, Florin NC, Scheiff AB, Müller CE. (2009) 1-alkyl-8-(piperazine-1-sulfonyl)phenylxanthines: development and characterization of adenosine A2B receptor antagonists and a new radioligand with subnanomolar affinity and subtype specificity. J Med Chem, 52 (13): 3994-4006. [PMID:19569717]
14. Bosch MP, Campos F, Niubó I, Rosell G, Díaz JL, Brea J, Loza MI, Guerrero A. (2004) Synthesis and biological activity of new potential agonists for the human adenosine A2A receptor. J Med Chem, 47 (16): 4041-53. [PMID:15267242]
15. Carroll MA, Doumad AB, Li J, Cheng MK, Falck JR, McGiff JC. (2006) Adenosine2A receptor vasodilation of rat preglomerular microvessels is mediated by EETs that activate the cAMP/PKA pathway. Am J Physiol Renal Physiol, 291 (1): F155-61. [PMID:16478979]
16. Chang LC, von Frijtag Drabbe Künzel JK, Mulder-Krieger T, Westerhout J, Spangenberg T, Brussee J, Ijzerman AP. (2007) 2,6,8-trisubstituted 1-deazapurines as adenosine receptor antagonists. J Med Chem, 50 (4): 828-34. [PMID:17300165]
17. Chen JF. (2003) The adenosine A(2A) receptor as an attractive target for Parkinson's disease treatment. Drug News Perspect, 16 (9): 597-604. [PMID:14702141]
18. Chen JF, Beilstein M, Xu YH, Turner TJ, Moratalla R, Standaert DG, Aloyo VJ, Fink JS, Schwarzschild MA. (2000) Selective attenuation of psychostimulant-induced behavioral responses in mice lacking A(2A) adenosine receptors. Neuroscience, 97 (1): 195-204. [PMID:10771351]
19. Chen JF, Huang Z, Ma J, Zhu J, Moratalla R, Standaert D, Moskowitz MA, Fink JS, Schwarzschild MA. (1999) A(2A) adenosine receptor deficiency attenuates brain injury induced by transient focal ischemia in mice. J Neurosci, 19 (21): 9192-200. [PMID:10531422]
20. Cheng RKY, Segala E, Robertson N, Deflorian F, Doré AS, Errey JC, Fiez-Vandal C, Marshall FH, Cooke RM. (2017) Structures of Human A1 and A2A Adenosine Receptors with Xanthines Reveal Determinants of Selectivity. Structure, 25 (8): 1275-1285.e4. [PMID:28712806]
21. Christofi FL, Zhang H, Yu JG, Guzman J, Xue J, Kim M, Wang YZ, Cooke HJ. (2001) Differential gene expression of adenosine A1, A2a, A2b, and A3 receptors in the human enteric nervous system. J Comp Neurol, 439 (1): 46-64. [PMID:11579381]
22. Chu YY, Tu KH, Lee YC, Kuo ZJ, Lai HL, Chern Y. (1996) Characterization of the rat A2a adenosine receptor gene. DNA Cell Biol, 15 (4): 329-37. [PMID:8639269]
23. Coney AM, Marshall JM. (1998) Role of adenosine and its receptors in the vasodilatation induced in the cerebral cortex of the rat by systemic hypoxia. J Physiol (Lond.), 509 ( Pt 2): 507-18. [PMID:9575299]
24. Congreve M, Andrews SP, Doré AS, Hollenstein K, Hurrell E, Langmead CJ, Mason JS, Ng IW, Tehan B, Zhukov A et al.. (2012) Discovery of 1,2,4-triazine derivatives as adenosine A(2A) antagonists using structure based drug design. J Med Chem, 55 (5): 1898-903. [PMID:22220592]
25. Cooper JA, Hill SJ, Alexander SP, Rubin PC, Horn EH. (1995) Adenosine receptor-induced cyclic AMP generation and inhibition of 5-hydroxytryptamine release in human platelets. Br J Clin Pharmacol, 40 (1): 43-50. [PMID:8527267]
26. Corsi C, Melani A, Bianchi L, Pepeu G, Pedata F. (1999) Striatal A2A adenosine receptors differentially regulate spontaneous and K+-evoked glutamate release in vivo in young and aged rats. Neuroreport, 10: 687-691. [PMID:10208531]
27. Daly JW, Hide I, Müller CE, Shamim M. (1991) Caffeine analogs: structure-activity relationships at adenosine receptors. Pharmacology, 42 (6): 309-21. [PMID:1658821]
28. Daly JW, Padgett WL, Secunda SI, Thompson RD, Olsson RA. (1993) Structure-activity relationships for 2-substituted adenosines at A1 and A2 adenosine receptors. Pharmacology, 46 (2): 91-100. [PMID:8441759]
29. de Lera Ruiz M, Lim YH, Zheng J. (2014) Adenosine A2A receptor as a drug discovery target. J Med Chem, 57 (9): 3623-50. [PMID:24164628]
30. Dickenson JM, Reeder S, Rees B, Alexander S, Kendall D. (2003) Functional expression of adenosine A2A and A3 receptors in the mouse dendritic cell line XS-106. Eur J Pharmacol, 474 (1): 43-51. [PMID:12909194]
31. Dionisotti S, Ferrara S, Molta C, Zocchi C, Ongini E. (1996) Labeling of A2A adenosine receptors in human platelets by use of the new nonxanthine antagonist radioligand [3H]SCH 58261. J Pharmacol Exp Ther, 278 (3): 1209-14. [PMID:8819504]
32. Dionisotti S, Ongini E, Zocchi C, Kull B, Arslan G, Fredholm BB. (1997) Characterization of human A2A adenosine receptors with the antagonist radioligand [3H]-SCH 58261. Br J Pharmacol, 121 (3): 353-60. [PMID:9179373]
33. Donoso MV, Aedo F, Huidobro-Toro JP. (2006) The role of adenosine A2A and A3 receptors on the differential modulation of norepinephrine and neuropeptide Y release from peripheral sympathetic nerve terminals. J Neurochem, 96 (6): 1680-95. [PMID:16539684]
34. Eastwood P, Gonzalez J, Paredes S, Nueda A, Domenech T, Alberti J, Vidal B. (2010) Discovery of N-(5,6-diarylpyridin-2-yl)amide derivatives as potent and selective A(2B) adenosine receptor antagonists. Bioorg Med Chem Lett, 20 (5): 1697-700. [PMID:20137946]
35. El Yacoubi M, Ledent C, Parmentier M, Daoust M, Costentin J, Vaugeois J. (2001) Absence of the adenosine A(2A) receptor or its chronic blockade decrease ethanol withdrawal-induced seizures in mice. Neuropharmacology, 40 (3): 424-32. [PMID:11166335]
36. Elzein E, Kalla RV, Li X, Perry T, Gimbel A, Zeng D, Lustig D, Leung K, Zablocki J. (2008) Discovery of a novel A2B adenosine receptor antagonist as a clinical candidate for chronic inflammatory airway diseases. J Med Chem, 51 (7): 2267-78. [PMID:18321039]
37. Elzein E, Zablocki J. (2008) A1 adenosine receptor agonists and their potential therapeutic applications. Expert Opin Investig Drugs, 17 (12): 1901-10. [PMID:19012505]
38. Engelen DP, Koopman JP, van der Brink ME, Bakker MH, Stadhouders AM, de Boer H. (1990) Differences in the intestinal microflora of normal and dystrophic BIO 8262 Nij Syrian hamsters. Z Versuchstierkd, 33 (2): 91-6. [PMID:2353549]
39. Fredholm BB. (1995) Astra Award Lecture. Adenosine, adenosine receptors and the actions of caffeine. Pharmacol Toxicol, 76 (2): 93-101. [PMID:7746802]
40. Fredholm BB, Chen JF, Masino SA, Vaugeois JM. (2005) Actions of adenosine at its receptors in the CNS: insights from knockouts and drugs. Annu Rev Pharmacol Toxicol, 45: 385-412. [PMID:15822182]
41. Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE. (2011) International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol Rev, 63 (1): 1-34. [PMID:21303899]
42. Fresco P, Diniz C, Gonçalves J. (2004) Facilitation of noradrenaline release by activation of adenosine A(2A) receptors triggers both phospholipase C and adenylate cyclase pathways in rat tail artery. Cardiovasc Res, 63 (4): 739-46. [PMID:15306230]
43. Furlong TJ, Pierce KD, Selbie LA, Shine J. (1992) Molecular characterization of a human brain adenosine A2 receptor. Brain Res Mol Brain Res, 15 (1-2): 62-6. [PMID:1331670]
44. Gao ZG, Jiang Q, Jacobson KA, Ijzerman AP. (2000) Site-directed mutagenesis studies of human A(2A) adenosine receptors: involvement of glu(13) and his(278) in ligand binding and sodium modulation. Biochem Pharmacol, 60 (5): 661-8. [PMID:10927024]
45. Gao ZG, Mamedova LK, Chen P, Jacobson KA. (2004) 2-Substituted adenosine derivatives: affinity and efficacy at four subtypes of human adenosine receptors. Biochem Pharmacol, 68 (10): 1985-93. [PMID:15476669]
46. Garber K. (2017) Adenosine checkpoint agent blazes a trail, joins immunotherapy roster. Nat Biotechnol, 35 (9): 805-807. [PMID:28898225]
47. García-Nafría J, Lee Y, Bai X, Carpenter B, Tate CG. (2018) Cryo-EM structure of the adenosine A2A receptor coupled to an engineered heterotrimeric G protein. Elife, 7. [PMID:29726815]
48. Gillespie RJ, Bamford SJ, Botting R, Comer M, Denny S, Gaur S, Griffin M, Jordan AM, Knight AR, Lerpiniere J et al.. (2009) Antagonists of the human A(2A) adenosine receptor. 4. Design, synthesis, and preclinical evaluation of 7-aryltriazolo[4,5-d]pyrimidines. J Med Chem, 52 (1): 33-47. [PMID:19072055]
49. Gomez JAC, Laria JCC-P. (2011) 4-aminopyrimidine derivatives and their as as adenosine A2A receptor antagonists. Patent number: WO2011121418A1. Assignee: Palobiofarma, S.L.. Priority date: 31/03/2010. Publication date: 06/10/2011.
50. Guo D, Mulder-Krieger T, IJzerman AP, Heitman LH. (2012) Functional efficacy of adenosine A₂A receptor agonists is positively correlated to their receptor residence time. Br J Pharmacol, 166 (6): 1846-59. [PMID:22324512]
51. Holschbach MH, Olsson RA, Bier D, Wutz W, Sihver W, Schüller M, Palm B, Coenen HH. (2002) Synthesis and evaluation of no-carrier-added 8-cyclopentyl-3-(3-[(18)F]fluoropropyl)-1-propylxanthine ([(18)F]CPFPX): a potent and selective A(1)-adenosine receptor antagonist for in vivo imaging. J Med Chem, 45 (23): 5150-6. [PMID:12408725]
52. Huang ZL, Qu WM, Eguchi N, Chen JF, Schwarzschild MA, Fredholm BB, Urade Y, Hayaishi O. (2005) Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci, 8 (7): 858-9. [PMID:15965471]
53. Jacobson KA IJzerman AP, Linden J. (1999) 1,3-Dialkylxanthine derivatives having high potency as antagonists at human A2B adenosine receptors. Drug Dev Res, (47): 45-53.
54. Jacobson KA, Gallo-Rodriguez C, Melman N, Fischer B, Maillard M, van Bergen A, van Galen PJ, Karton Y. (1993) Structure-activity relationships of 8-styrylxanthines as A2-selective adenosine antagonists. J Med Chem, 36 (10): 1333-42. [PMID:8496902]
55. Jacobson KA, Gao ZG. (2006) Adenosine receptors as therapeutic targets. Nat Rev Drug Discov, 5 (3): 247-64. [PMID:16518376]
56. Jarvis MF, Schulz R, Hutchison AJ, Do UH, Sills MA, Williams M. (1989) [3H]CGS 21680, a selective A2 adenosine receptor agonist directly labels A2 receptors in rat brain. J Pharmacol Exp Ther, 251 (3): 888-93. [PMID:2600819]
57. Jenner P. (2003) A2A antagonists as novel non-dopaminergic therapy for motor dysfunction in PD. Neurology, 61 (11 Suppl 6): S32-8. [PMID:14663007]
58. Jenner P. (2005) Istradefylline, a novel adenosine A2A receptor antagonist, for the treatment of Parkinson's disease. Expert Opin Investig Drugs, 14 (6): 729-38. [PMID:16004599]
59. Kalk P, Eggert B, Relle K, Godes M, Heiden S, Sharkovska Y, Fischer Y, Ziegler D, Bielenberg GW, Hocher B. (2007) The adenosine A1 receptor antagonist SLV320 reduces myocardial fibrosis in rats with 5/6 nephrectomy without affecting blood pressure. Br J Pharmacol, 151 (7): 1025-32. [PMID:17558436]
60. Karton Y, Jiang JL, Ji XD, Melman N, Olah ME, Stiles GL, Jacobson KA. (1996) Synthesis and biological activities of flavonoid derivatives as A3 adenosine receptor antagonists. J Med Chem, 39 (12): 2293-301. [PMID:8691424]
61. Kiesman WF, Zhao J, Conlon PR, Dowling JE, Petter RC, Lutterodt F, Jin X, Smits G, Fure M, Jayaraj A et al.. (2006) Potent and orally bioavailable 8-bicyclo[2.2.2]octylxanthines as adenosine A1 receptor antagonists. J Med Chem, 49 (24): 7119-31. [PMID:17125264]
62. Kiesman WF, Zhao J, Conlon PR, Petter RC, Jin X, Smits G, Lutterodt F, Sullivan GW, Linden J. (2006) Norbornyllactone-substituted xanthines as adenosine A(1) receptor antagonists. Bioorg Med Chem, 14 (11): 3654-61. [PMID:16458010]
63. Kim J, Wess J, van Rhee AM, Schöneberg T, Jacobson KA. (1995) Site-directed mutagenesis identifies residues involved in ligand recognition in the human A2a adenosine receptor. J Biol Chem, 270 (23): 13987-97. [PMID:7775460]
64. Kim SA, Marshall MA, Melman N, Kim HS, Müller CE, Linden J, Jacobson KA. (2002) Structure-activity relationships at human and rat A2B adenosine receptors of xanthine derivatives substituted at the 1-, 3-, 7-, and 8-positions. J Med Chem, 45 (11): 2131-8. [PMID:12014951]
65. Kim YC, Ji X, Melman N, Linden J, Jacobson KA. (2000) Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A(2B) adenosine receptors. J Med Chem, 43 (6): 1165-72. [PMID:10737749]
66. Klotz KN, Falgner N, Kachler S, Lambertucci C, Vittori S, Volpini R, Cristalli G. (2007) [3H]HEMADO--a novel tritiated agonist selective for the human adenosine A3 receptor. Eur J Pharmacol, 556 (1-3): 14-8. [PMID:17126322]
67. Klotz KN, Hessling J, Hegler J, Owman C, Kull B, Fredholm BB, Lohse MJ. (1998) Comparative pharmacology of human adenosine receptor subtypes - characterization of stably transfected receptors in CHO cells. Naunyn Schmiedebergs Arch Pharmacol, 357 (1): 1-9. [PMID:9459566]
68. Kull B, Arslan G, Nilsson C, Owman C, Lorenzen A, Schwabe U, Fredholm BB. (1999) Differences in the order of potency for agonists but not antagonists at human and rat adenosine A2A receptors. Biochem Pharmacol, 57 (1): 65-75. [PMID:9920286]
69. Kull B, Svenningsson P, Fredholm BB. (2000) Adenosine A(2A) receptors are colocalized with and activate g(olf) in rat striatum. Mol Pharmacol, 58 (4): 771-7. [PMID:10999947]
70. Kuno A, Critz SD, Cui L, Solodushko V, Yang XM, Krahn T, Albrecht B, Philipp S, Cohen MV, Downey JM. (2007) Protein kinase C protects preconditioned rabbit hearts by increasing sensitivity of adenosine A2b-dependent signaling during early reperfusion. J Mol Cell Cardiol, 43 (3): 262-71. [PMID:17632123]
71. Lappas CM, Rieger JM, Linden J. (2005) A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol, 174 (2): 1073-80. [PMID:15634932]
72. Le F, Townsend-Nicholson A, Baker E, Sutherland GR, Schofield PR. (1996) Characterization and chromosomal localization of the human A2a adenosine receptor gene: ADORA2A. Biochem Biophys Res Commun, 223 (2): 461-7. [PMID:8670304]
73. Lebon G, Warne T, Edwards PC, Bennett K, Langmead CJ, Leslie AG, Tate CG. (2011) Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature, 474 (7352): 521-5. [PMID:21593763]
74. Lebon G, Warne T, Edwards PC, Bennett K, Langmead CJ, Leslie AG, Tate CG. (2011) Agonist-bound adenosine A2A receptor structures reveal common features of GPCR activation. Nature, 474 (7352): 521-5. [PMID:21593763]
75. Ledent C, Vaugeois JM, Schiffmann SN, Pedrazzini T, El Yacoubi M, Vanderhaeghen JJ, Costentin J, Heath JK, Vassart G, Parmentier M. (1997) Aggressiveness, hypoalgesia and high blood pressure in mice lacking the adenosine A2a receptor. Nature, 388 (6643): 674-8. [PMID:9262401]
76. Leone RD, Lo YC, Powell JD. (2015) A2aR antagonists: Next generation checkpoint blockade for cancer immunotherapy. Comput Struct Biotechnol J, 13: 265-72. [PMID:25941561]
77. Li L, Hao JX, Fredholm BB, Schulte G, Wiesenfeld-Hallin Z, Xu XJ. (2010) Peripheral adenosine A2A receptors are involved in carrageenan-induced mechanical hyperalgesia in mice. Neuroscience, 170 (3): 923-8. [PMID:20678550]
78. Li W, Dai S, An J, Xiong R, Li P, Chen X, Zhao Y, Liu P, Wang H, Zhu P et al.. (2009) Genetic inactivation of adenosine A2A receptors attenuates acute traumatic brain injury in the mouse cortical impact model. Exp Neurol, 215 (1): 69-76. [PMID:18938161]
79. Liang BT, Urso R, Sambraski E, Jacobson KA. (2010) . In A3 Adenosine Receptors from Cell Biology to Pharmacology and Therapeutics. Edited by Borea PA (Springer) . [ISBN:9789048131440]
80. Liu W, Chun E, Thompson AA, Chubukov P, Xu F, Katritch V, Han GW, Roth CB, Heitman LH, IJzerman AP et al.. (2012) Structural basis for allosteric regulation of GPCRs by sodium ions. Science, 337 (6091): 232-6. [PMID:22798613]
81. Lopes LV, Halldner L, Rebola N, Johansson B, Ledent C, Chen JF, Fredholm BB, Cunha RA. (2004) Binding of the prototypical adenosine A(2A) receptor agonist CGS 21680 to the cerebral cortex of adenosine A(1) and A(2A) receptor knockout mice. Br J Pharmacol, 141: 1006-1014. [PMID:14993095]
82. Müller CE, Stein B. (1996) Adenosine receptor antagonists: structures and potential therapeutic applications. Curr Pharm Des, 2: 501-530.
83. Maddock HL, Mocanu MM, Yellon DM. (2002) Adenosine A(3) receptor activation protects the myocardium from reperfusion/reoxygenation injury. Am J Physiol Heart Circ Physiol, 283 (4): H1307-13. [PMID:12234780]
84. Marks GA, Shaffery JP, Speciale SG, Birabil CG. (2003) Enhancement of rapid eye movement sleep in the rat by actions at A1 and A2a adenosine receptor subtypes with a differential sensitivity to atropine. Neuroscience, 116 (3): 913-20. [PMID:12573729]
85. Marquardt DL, Walker LL, Heinemann S. (1994) Cloning of two adenosine receptor subtypes from mouse bone marrow-derived mast cells. J Immunol, 152 (9): 4508-15. [PMID:8157966]
86. McPherson JA, Barringhaus KG, Bishop GG, Sanders JM, Rieger JM, Hesselbacher SE, Gimple LW, Powers ER, Macdonald T, Sullivan G et al.. (2001) Adenosine A(2A) receptor stimulation reduces inflammation and neointimal growth in a murine carotid ligation model. Arterioscler Thromb Vasc Biol, 21 (5): 791-6. [PMID:11348876]
87. Mediavilla-Varela M, Castro J, Chiappori A, Noyes D, Hernandez DC, Allard B, Stagg J, Antonia SJ. (2017) A Novel Antagonist of the Immune Checkpoint Protein Adenosine A2a Receptor Restores Tumor-Infiltrating Lymphocyte Activity in the Context of the Tumor Microenvironment. Neoplasia, 19 (7): 530-536. [PMID:28582704]
88. Melman A, Gao ZG, Kumar D, Wan TC, Gizewski E, Auchampach JA, Jacobson KA. (2008) Design of (N)-methanocarba adenosine 5'-uronamides as species-independent A3 receptor-selective agonists. Bioorg Med Chem Lett, 18 (9): 2813-9. [PMID:18424135]
89. Minetti P, Tinti MO, Carminati P, Castorina M, Di Cesare MA, Di Serio S, Gallo G, Ghirardi O, Giorgi F, Giorgi L et al.. (2005) 2-n-Butyl-9-methyl-8-[1,2,3]triazol-2-yl-9H-purin-6-ylamine and analogues as A2A adenosine receptor antagonists. Design, synthesis, and pharmacological characterization. J Med Chem, 48 (22): 6887-96. [PMID:16250647]
90. Montesinos MC, Desai A, Chen JF, Yee H, Schwarzschild MA, Fink JS, Cronstein BN. (2002) Adenosine promotes wound healing and mediates angiogenesis in response to tissue injury via occupancy of A(2A) receptors. Am J Pathol, 160 (6): 2009-18. [PMID:12057906]
91. Murray RD, Churchill PC. (1984) Effects of adenosine receptor agonists in the isolated, perfused rat kidney. Am J Physiol, 247 (3 Pt 2): H343-8. [PMID:6089592]
92. Müller CE, Ferré S. (2007) Blocking striatal adenosine A2A receptors: a new strategy for basal ganglia disorders. Recent Pat CNS Drug Discov, 2 (1): 1-21. [PMID:18221214]
93. Müller CE, Jacobson KA. (2011) Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim Biophys Acta, 1808 (5): 1290-308. [PMID:21185259]
94. Müller CE, Maurinsh J, Sauer R. (2000) Binding of [3H]MSX-2 (3-(3-hydroxypropyl)-7-methyl-8-(m-methoxystyryl)-1-propargylxanthine) to rat striatal membranes--a new, selective antagonist radioligand for A(2A) adenosine receptors. Eur J Pharm Sci, 10 (4): 259-65. [PMID:10838015]
95. Müller CE, Shi D, Manning M, Daly JW. (1993) Synthesis of paraxanthine analogs (1,7-disubstituted xanthines) and other xanthines unsubstituted at the 3-position: structure-activity relationships at adenosine receptors. J Med Chem, 36 (22): 3341-9. [PMID:8230124]
96. Müller CE, Thorand M, Qurishi R, Diekmann M, Jacobson KA, Padgett WL, Daly JW. (2002) Imidazo[2,1-i]purin-5-ones and related tricyclic water-soluble purine derivatives: potent A(2A)- and A(3)-adenosine receptor antagonists. J Med Chem, 45 (16): 3440-50. [PMID:12139454]
97. Naassila M, Ledent C, Daoust M. (2002) Low ethanol sensitivity and increased ethanol consumption in mice lacking adenosine A2A receptors. J Neurosci, 22 (23): 10487-93. [PMID:12451148]
98. Nagel J, Schladebach H, Koch M, Schwienbacher I, Müller CE, Hauber W. (2003) Effects of an adenosine A2A receptor blockade in the nucleus accumbens on locomotion, feeding, and prepulse inhibition in rats. Synapse, 49 (4): 279-86. [PMID:12827647]
99. Ngai AC, Coyne EF, Meno JR, West GA, Winn HR. (2001) Receptor subtypes mediating adenosine-induced dilation of cerebral arterioles. Am J Physiol Heart Circ Physiol, 280 (5): H2329-35. [PMID:11299238]
100. Nowak M, Lynch L, Yue S, Ohta A, Sitkovsky M, Balk SP, Exley MA. (2010) The A2aR adenosine receptor controls cytokine production in iNKT cells. Eur J Immunol, 40 (3): 682-7. [PMID:20039304]
101. Obiefuna PC, Batra VK, Nadeem A, Borron P, Wilson CN, Mustafa SJ. (2005) A novel A1 adenosine receptor antagonist, L-97-1 [3-[2-(4-aminophenyl)-ethyl]-8-benzyl-7-{2-ethyl-(2-hydroxy-ethyl)-amino]-ethyl}-1-propyl-3,7-dihydro-purine-2,6-dione], reduces allergic responses to house dust mite in an allergic rabbit model of asthma. J Pharmacol Exp Ther, 315 (1): 329-36. [PMID:16020631]
102. Ohta A, Sitkovsky M. (2001) Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature, 414 (6866): 916-20. [PMID:11780065]
103. Olah ME. (1997) Identification of A2a adenosine receptor domains involved in selective coupling to Gs. Analysis of chimeric A1/A2a adenosine receptors. J Biol Chem, 272 (1): 337-44. [PMID:8995267]
104. Ongini E, Dionisotti S, Gessi S, Irenius E, Fredholm BB. (1999) Comparison of CGS 15943, ZM 241385 and SCH 58261 as antagonists at human adenosine receptors. Naunyn Schmiedebergs Arch Pharmacol, 359 (1): 7-10. [PMID:9933143]
105. Ozola V, Thorand M, Diekmann M, Qurishi R, Schumacher B, Jacobson KA, Müller CE. (2003) 2-Phenylimidazo[2,1-i]purin-5-ones: structure-activity relationships and characterization of potent and selective inverse agonists at Human A3 adenosine receptors. Bioorg Med Chem, 11 (3): 347-56. [PMID:12517430]
106. Palmer TM, Poucher SM, Jacobson KA, Stiles GL. (1995) 125I-4-(2-[7-amino-2-[2-furyl][1,2,4]triazolo[2,3-a][1,3,5] triazin-5-yl-amino]ethyl)phenol, a high affinity antagonist radioligand selective for the A2a adenosine receptor. Mol Pharmacol, 48 (6): 970-4. [PMID:8848012]
107. Peirce SM, Skalak TC, Rieger JM, Macdonald TL, Linden J. (2001) Selective A(2A) adenosine receptor activation reduces skin pressure ulcer formation and inflammation. Am J Physiol Heart Circ Physiol, 281 (1): H67-74. [PMID:11406470]
108. Peterfreund RA, MacCollin M, Gusella J, Fink JS. (1996) Characterization and expression of the human A2a adenosine receptor gene. J Neurochem, 66 (1): 362-8. [PMID:8522976]
109. Pfeiffer M, Kirscht S, Stumm R, Koch T, Wu D, Laugsch M, Schröder H, Höllt V, Schulz S. (2003) Heterodimerization of substance P and mu-opioid receptors regulates receptor trafficking and resensitization. J Biol Chem, 278 (51): 51630-7. [PMID:14532289]
110. Pfister JR, Belardinelli L, Lee G, Lum RT, Milner P, Stanley WC, Linden J, Baker SP, Schreiner G. (1997) Synthesis and biological evaluation of the enantiomers of the potent and selective A1-adenosine antagonist 1,3-dipropyl-8-[2-(5,6-epoxynorbonyl)]-xanthine. J Med Chem, 40 (12): 1773-8. [PMID:9191953]
111. Pinna A, Wardas J, Simola N, Morelli M. (2005) New therapies for the treatment of Parkinson's disease: adenosine A2A receptor antagonists. Life Sci, 77 (26): 3259-67. [PMID:15979104]
112. Popoli P, Betto P, Reggio R, Ricciarello G. (1995) Adenosine A2A receptor stimulation enhances striatal extracellular glutamate levels in rats. Eur J Pharmacol, 287 (2): 215-7. [PMID:8749040]
113. Pretorius J, Malan SF, Castagnoli N, Bergh JJ, Petzer JP. (2008) Dual inhibition of monoamine oxidase B and antagonism of the adenosine A(2A) receptor by (E,E)-8-(4-phenylbutadien-1-yl)caffeine analogues. Bioorg Med Chem, 16 (18): 8676-84. [PMID:18723354]
114. Rebola N, Canas PM, Oliveira CR, Cunha RA. (2005) Different synaptic and subsynaptic localization of adenosine A2A receptors in the hippocampus and striatum of the rat. Neuroscience, 132 (4): 893-903. [PMID:15857695]
115. Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J. (1998) Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol, 401 (2): 163-86. [PMID:9822147]
116. Saki M, Tsumuki H, Nonaka H, Shimada J, Ichimura M. (2002) KF26777 (2-(4-bromophenyl)-7,8-dihydro-4-propyl-1H-imidazo[2,1-i]purin-5(4H)-one dihydrochloride), a new potent and selective adenosine A3 receptor antagonist. Eur J Pharmacol, 444 (3): 133-41. [PMID:12063073]
117. Salvatore CA, Jacobson MA, Taylor HE, Linden J, Johnson RG. (1993) Molecular cloning and characterization of the human A3 adenosine receptor. Proc Natl Acad Sci USA, 90 (21): 10365-9. [PMID:8234299]
118. Satoh S, Matsumura H, Hayaishi O. (1998) Involvement of adenosine A2A receptor in sleep promotion. Eur J Pharmacol, 351 (2): 155-62. [PMID:9686998]
119. Satoh S, Matsumura H, Koike N, Tokunaga Y, Maeda T, Hayaishi O. (1999) Region-dependent difference in the sleep-promoting potency of an adenosine A2A receptor agonist. Eur J Neurosci, 11 (5): 1587-97. [PMID:10215911]
120. Satoh S, Matsumura H, Suzuki F, Hayaishi O. (1996) Promotion of sleep mediated by the A2a-adenosine receptor and possible involvement of this receptor in the sleep induced by prostaglandin D2 in rats. Proc Natl Acad Sci USA, 93 (12): 5980-4. [PMID:8650205]
121. Sauer R, Maurinsh J, Reith U, Fülle F, Klotz KN, Müller CE. (2000) Water-soluble phosphate prodrugs of 1-propargyl-8-styrylxanthine derivatives, A(2A)-selective adenosine receptor antagonists. J Med Chem, 43 (3): 440-8. [PMID:10669571]
122. Scammell TE, Gerashchenko DY, Mochizuki T, McCarthy MT, Estabrooke IV, Sears CA, Saper CB, Urade Y, Hayaishi O. (2001) An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience, 107 (4): 653-63. [PMID:11720788]
123. Schnurr M, Toy T, Shin A, Hartmann G, Rothenfusser S, Soellner J, Davis ID, Cebon J, Maraskovsky E. (2004) Role of adenosine receptors in regulating chemotaxis and cytokine production of plasmacytoid dendritic cells. Blood, 103 (4): 1391-7. [PMID:14551144]
124. Schulte G, Fredholm BB. (2000) Human adenosine A(1), A(2A), A(2B), and A(3) receptors expressed in Chinese hamster ovary cells all mediate the phosphorylation of extracellular-regulated kinase 1/2. Mol Pharmacol, 58 (3): 477-82. [PMID:10953039]
125. Seitz L, Jin L, Leleti M, Ashok D, Jeffrey J, Rieger A, Tiessen RG, Arold G, Tan JBL, Powers JP et al.. (2019) Safety, tolerability, and pharmacology of AB928, a novel dual adenosine receptor antagonist, in a randomized, phase 1 study in healthy volunteers. Invest New Drugs, 37 (4): 711-721. [PMID:30569245]
126. Shen HY, Coelho JE, Ohtsuka N, Canas PM, Day YJ, Huang QY, Rebola N, Yu L, Boison D, Cunha RA et al.. (2008) A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knock-outs. J Neurosci, 28 (12): 2970-5. [PMID:18354001]
127. Shimada J, Koike N Nonaka H, Shiozaki S, Yanagawa K, Kanda T, Kobayashi H, Ichimura M, Nakamura J, Kase H et al.. (1997) Adenosine A2A antagonists with potent anti-cataleptic activity. Bioorg Med Chem Lett, (7): 2349-2352.
128. Shinkre BA, Kumar TS, Gao ZG, Deflorian F, Jacobson KA, Trenkle WC. (2010) Synthesis and evaluation of 1,2,4-triazolo[1,5-c]pyrimidine derivatives as A2A receptor-selective antagonists. Bioorg Med Chem Lett, 20 (19): 5690-4. [PMID:20801028]
129. Shiriaeva A, Park D, Kim G, Lee Y, Hou X, Jarhad DB, Kim G, Yu J, Hyun YE, Kim W et al.. (2022) GPCR Agonist-to-Antagonist Conversion: Enabling the Design of Nucleoside Functional Switches for the A2A Adenosine Receptor. J Med Chem, 65 (17): 11648-11657. [PMID:35977382]
130. Smailagic A, Hakansson H, Jansson A, Michaalsson E, Lal H, Uddin M, Catley M. (2014) Abstract A5742: Impact of A2a receptor activation on inflammation, lung mechanics and a wide panel of mediators in the LPS-induced lung injury in mice. Am J Respir Crit Care Med, 189: A5742.
131. Talukder MA, Morrison RR, Ledent C, Mustafa SJ. (2003) Endogenous adenosine increases coronary flow by activation of both A2A and A2B receptors in mice. J Cardiovasc Pharmacol, 41 (4): 562-70. [PMID:12658057]
132. Todde S, Moresco RM, Simonelli P, Baraldi PG, Cacciari B, Spalluto G, Varani K, Monopoli A, Matarrese M, Carpinelli A et al.. (2000) Design, radiosynthesis, and biodistribution of a new potent and selective ligand for in vivo imaging of the adenosine A(2A) receptor system using positron emission tomography. J Med Chem, 43 (23): 4359-62. [PMID:11087559]
133. van Muijlwijk-Koezen JE, Timmerman H, Link R, van der Goot H, IJzerman AP. (1998) A novel class of adenosine A3 receptor ligands. 1. 3-(2-Pyridinyl)isoquinoline derivatives. J Med Chem, 41 (21): 3987-93. [PMID:9767636]
134. Varani K, Gessi S, Dalpiaz A, Borea PA. (1996) Pharmacological and biochemical characterization of purified A2a adenosine receptors in human platelet membranes by [3H]-CGS 21680 binding. Br J Pharmacol, 117 (8): 1693-701. [PMID:8732278]
135. Varani K, Gessi S, Merighi S, Vincenzi F, Cattabriga E, Benini A, Klotz KN, Baraldi PG, Tabrizi MA, Lennan SM et al.. (2005) Pharmacological characterization of novel adenosine ligands in recombinant and native human A2B receptors. Biochem Pharmacol, 70 (11): 1601-12. [PMID:16219300]
136. Varani K, Merighi S, Gessi S, Klotz KN, Leung E, Baraldi PG, Cacciari B, Romagnoli R, Spalluto G, Borea PA. (2000) [(3)H]MRE 3008F20: a novel antagonist radioligand for the pharmacological and biochemical characterization of human A(3) adenosine receptors. Mol Pharmacol, 57 (5): 968-75. [PMID:10779381]
137. Varani K, Portaluppi F, Gessi S, Merighi S, Ongini E, Belardinelli L, Borea PA. (2000) Dose and time effects of caffeine intake on human platelet adenosine A(2A) receptors : functional and biochemical aspects. Circulation, 102 (3): 285-9. [PMID:10899090]
138. Varani K, Portaluppi F, Merighi S, Ongini E, Belardinelli L, Borea PA. (1999) Caffeine alters A2A adenosine receptors and their function in human platelets. Circulation, 99 (19): 2499-502. [PMID:10330379]
139. Varani K, Vincenzi F, Tosi A, Targa M, Masieri FF, Ongaro A, De Mattei M, Massari L, Borea PA. (2010) Expression and functional role of adenosine receptors in regulating inflammatory responses in human synoviocytes. Br J Pharmacol, 160 (1): 101-15. [PMID:20331607]
140. Vidal B, Nueda A, Esteve C, Domenech T, Benito S, Reinoso RF, Pont M, Calbet M, López R, Cadavid MI et al.. (2007) Discovery and characterization of 4'-(2-furyl)-N-pyridin-3-yl-4,5'-bipyrimidin-2'-amine (LAS38096), a potent, selective, and efficacious A2B adenosine receptor antagonist. J Med Chem, 50 (11): 2732-6. [PMID:17469811]
141. Vitzthum H, Weiss B, Bachleitner W, Krämer BK, Kurtz A. (2004) Gene expression of adenosine receptors along the nephron. Kidney Int, 65 (4): 1180-90. [PMID:15086457]
142. Volpini R, Costanzi S, Lambertucci C, Taffi S, Vittori S, Klotz KN, Cristalli G. (2002) N(6)-alkyl-2-alkynyl derivatives of adenosine as potent and selective agonists at the human adenosine A(3) receptor and a starting point for searching A(2B) ligands. J Med Chem, 45 (15): 3271-9. [PMID:12109910]
143. Wan W, Sutherland GR, Geiger JD. (1990) Binding of the adenosine A2 receptor ligand [3H]CGS 21680 to human and rat brain: evidence for multiple affinity sites. J Neurochem, 55 (5): 1763-71. [PMID:2213023]
144. Wang JH, Short J, Ledent C, Lawrence AJ, van den Buuse M. (2003) Reduced startle habituation and prepulse inhibition in mice lacking the adenosine A2A receptor. Behav Brain Res, 143 (2): 201-7. [PMID:12900046]
145. Weiss SM, Benwell K, Cliffe IA, Gillespie RJ, Knight AR, Lerpiniere J, Misra A, Pratt RM, Revell D, Upton R et al.. (2003) Discovery of nonxanthine adenosine A2A receptor antagonists for the treatment of Parkinson's disease. Neurology, 61 (11 Suppl 6): S101-6. [PMID:14663021]
146. Weyler S, Fülle F, Diekmann M, Schumacher B, Hinz S, Klotz KN, Müller CE. (2006) Improving potency, selectivity, and water solubility of adenosine A1 receptor antagonists: xanthines modified at position 3 and related pyrimido[1,2,3-cd]purinediones. ChemMedChem, 1 (8): 891-902. [PMID:16902942]
147. Willingham SB, Ho PY, Hotson A, Hill C, Piccione EC, Hsieh J, Liu L, Buggy JJ, McCaffery I, Miller RA. (2018) A2AR Antagonism with CPI-444 Induces Antitumor Responses and Augments Efficacy to Anti-PD-(L)1 and Anti-CTLA-4 in Preclinical Models. Cancer Immunol Res, 6 (10): 1136-1149. [PMID:30131376]
148. Xu F, Wu H, Katritch V, Han GW, Jacobson KA, Gao ZG, Cherezov V, Stevens RC. (2011) Structure of an agonist-bound human A2A adenosine receptor. Science, 332 (6027): 322-7. [PMID:21393508]
149. Yan L, Burbiel JC, Maass A, Müller CE. (2003) Adenosine receptor agonists: from basic medicinal chemistry to clinical development. Expert Opin Emerg Drugs, 8 (2): 537-76. [PMID:14662005]
150. Yang X, van Veldhoven JPD, Offringa J, Kuiper BJ, Lenselink EB, Heitman LH, van der Es D, IJzerman AP. (2019) Development of Covalent Ligands for G Protein-Coupled Receptors: A Case for the Human Adenosine A3 Receptor. J Med Chem, 62 (7): 3539-3552. DOI: 10.1021/acs.jmedchem.8b02026 [PMID:30869893]
151. Yu C, Gupta J, Chen JF, Yin HH. (2009) Genetic deletion of A2A adenosine receptors in the striatum selectively impairs habit formation. J Neurosci, 29 (48): 15100-3. [PMID:19955361]
152. Zhang N, Yang D, Dong H, Chen Q, Dimitrova DI, Rogers TJ, Sitkovsky M, Oppenheim JJ. (2006) Adenosine A2a receptors induce heterologous desensitization of chemokine receptors. Blood, 108 (1): 38-44. [PMID:16522819]
153. Zhou SJ, Zhu ME, Shu D, Du XP, Song XH, Wang XT, Zheng RY, Cai XH, Chen JF, He JC. (2009) Preferential enhancement of working memory in mice lacking adenosine A(2A) receptors. Brain Res, 1303: 74-83. [PMID:19785999]
















