|
Synonyms: 1-L-leucine-2-L-threonine-63-desulfohirudin | Refludan®
lepirudin is an approved drug (FDA (1998))
Compound class:
Peptide or derivative
Comment: Discontinued (not withdrawn). The drug is a recombinant form of hirudin, the salivary anticoagulant found in leeches (see UniProt P01050), but has two amino acid modifications; substitution of Leu1 for Ile and no sulfate group on Tyr63. The PubChem entry linked to above represents the chemical structure of Lepirudin.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: lepirudin |
Peptide Sequence  |
|
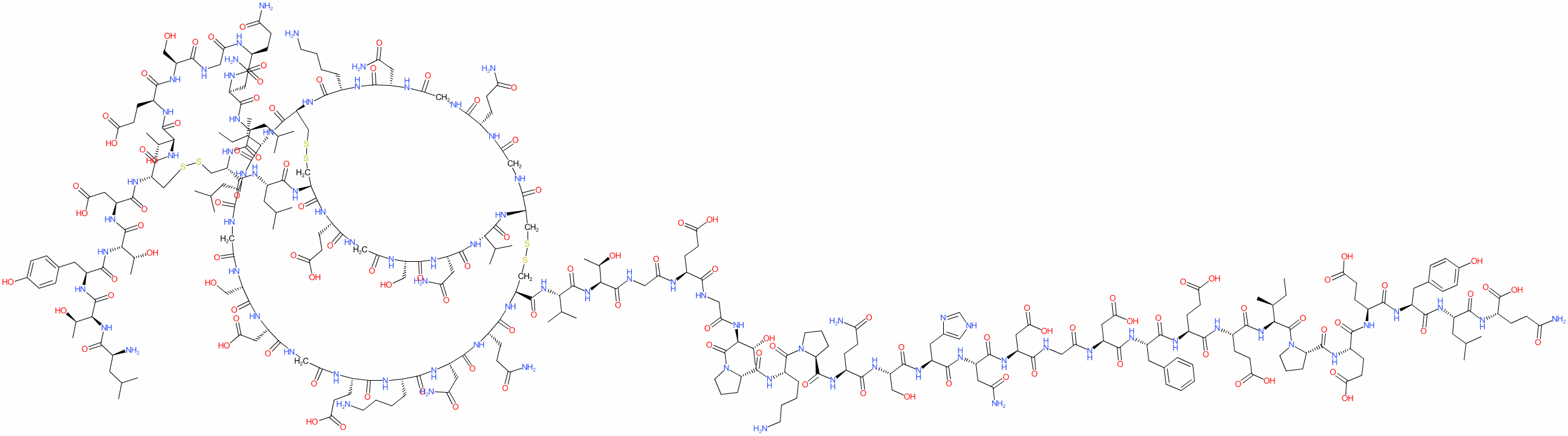
| LVYTDCTESGQNLCLCEGSNVCGQGNKCILGSDGEKNQCVTGEGTPKPQSHNDGDFEEIPEEYLQ | |
| H-Leu-Thr-Tyr-Thr-Asp-Cys(1)-Thr-Glu-Ser-Gly-Gln-Asn-Leu-Cys(1)-Leu-Cys(2)-Glu-Gly-Ser-Asn-Val-Cys(3)-Gly-Gln-Gly-Asn-Lys-Cys(2)-Ile-Leu-Gly-Ser-Asp-Gly-Glu-Lys-Asn-Gln-Cys(3)-Val-Thr-Gly-Glu-Gly-Thr-Pro-Lys-Pro-Gln-Ser-His-Asn-Asp-Gly-Asp-Phe-Glu-Glu-Ile-Pro-Glu-Glu-Tyr-Leu-Gln-OH | |
HELM Notation  |
|
| PEPTIDE1{L.T.Y.T.D.C.T.E.S.G.Q.N.L.C.L.C.E.G.S.N.V.C.G.Q.G.N.K.C.I.L.G.S.D.G.E.K.N.Q.C.V.T.G.E.G.T.P.K.P.Q.S.H.N.D.G.D.F.E.E.I.P.E.E.Y.L.Q}$PEPTIDE1,PEPTIDE1,6:R3-14:R3|PEPTIDE1,PEPTIDE1,16:R3-28:R3|PEPTIDE1,PEPTIDE1,22:R3-39:R3$$$ | |
| Post-translational Modification | |
| I1L at the N-terminal and absence of a sulfate group at Y63. | |
Download 2D Structure  |
|
| Canonical SMILES | Download |
| Isomeric SMILES | Download |
Molecular structure representations generated using Open Babel