|
Synonyms: MK 8776 | SCH 900776
Compound class:
Synthetic organic
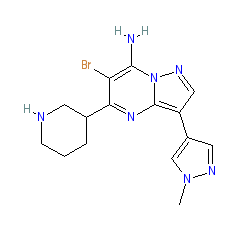
Comment: The discovery and synthesis of MK-8776 (a.k.a. SCH 900776) is described in [2], where it is compound 9b. In the article by Guzi et al (2011) [1], the compound is shown with an (R) stereo center (see PubChem CID 46239015), whereas it is shown with no stereochemistry by Labroli et al (2011). MK-8776 is primarily an inhibitor of checkpoint kinase 1 (CHK1), with minimal inhibitory activity on cyclin dependent kinase 2 (CDK2).
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Guzi TJ, Paruch K, Dwyer MP, Labroli M, Shanahan F, Davis N, Taricani L, Wiswell D, Seghezzi W, Penaflor E et al.. (2011)
Targeting the replication checkpoint using SCH 900776, a potent and functionally selective CHK1 inhibitor identified via high content screening. Mol Cancer Ther, 10 (4): 591-602. [PMID:21321066] |
|
2. Labroli M, Paruch K, Dwyer MP, Alvarez C, Keertikar K, Poker C, Rossman R, Duca JS, Fischmann TO, Madison V et al.. (2011)
Discovery of pyrazolo[1,5-a]pyrimidine-based CHK1 inhibitors: a template-based approach--part 2. Bioorg Med Chem Lett, 21 (1): 471-4. [PMID:21094607] |