|
Synonyms: compound 11c [PMID: 2617910] | Ro 09-4889
Compound class:
Synthetic organic
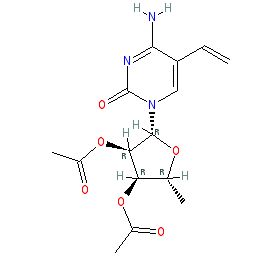
Comment: RO0094889 is a prodrug dihydropyrimidine dehydrogenase (DPD) inhibitor that was developed by Hoffmann-La Roche [3]. RO0094889 is metabolised by enzymes secreted by tumours to produce the DPD inhibitor 5-vinyluracil. This leads to inhibition of DPD-mediated catabolic degradation of the 5-fluorouracil produced locally in tumours from capecitabine [2]. RO0094889 is orally active.
|
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Bellibas SE, Patel I, Chamorey E, Brivet B, Bush ED, Kircher C, Nave S, Banken L, Renée N, Milano G. (2004)
Single ascending dose tolerability, pharmacokinetic-pharmacodynamic study of dihydropyrimidine dehydrogenase inhibitor Ro 09-4889. Clin Cancer Res, 10 (7): 2327-35. [PMID:15073108] |
|
2. Endo M, Miwa M, Eda H, Ura M, Tanimura H, Ishikawa T, Miyazaki-Nose T, Hattori K, Shimma N, Yamada-Okabe H et al.. (2003)
Augmentation of the antitumor activity of capecitabine by a tumor selective dihydropyrimidine dehydrogenase inhibitor, RO0094889. Int J Cancer, 106 (5): 799-805. [PMID:12866042] |
|
3. Hattori K, Kohchi Y, Oikawa N, Suda H, Ura M, Ishikawa T, Miwa M, Endoh M, Eda H, Tanimura H et al.. (2003)
Design and synthesis of the tumor-activated prodrug of dihydropyrimidine dehydrogenase (DPD) inhibitor, RO0094889 for combination therapy with capecitabine. Bioorg Med Chem Lett, 13 (5): 867-72. [PMID:12617910] |