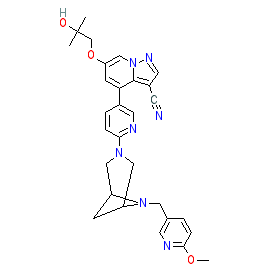
|
Synonyms: example 163 [WO2018071447A1] | LOXO-292 | LOXO292 | LY3527723 | Retevmo®
selpercatinib is an approved drug (FDA (2020), EMA (2021))
Compound class:
Synthetic organic
Comment: Selpercatinib (LOXO-292) is a potent, selective and orally bioavailable ATP-competitive RET kinase inhibitor that was developed by Loxo Oncology. It inhibits wild-type RET, RET fusions,and RET carrying activating mutations and acquired resistance mutations [5]. This reference by Subbiah et al. contains preclinical and proof-of-concept data for LOXO-292. This compound was rationally designed to offer a novel therapeutic option for heavily pretreated, multikinase inhibitor-experienced patients whose tumours carry diverse RET alterations.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| References |
|
1. Andrews SW, Aronow S, Blake JF, Brandhuber BJ, Cook A, Hass J, Jiang Y, Kolakowski GR, McFaddin EA, McKenney M et al.. (2018)
Substituted pyrazolo[1,5-a]pyridine compounds as ret kinase inhibitors. Patent number: WO2018071447A1. Assignee: Loxo Oncology. Priority date: 10/10/2016. Publication date: 19/04/2018. |
|
2. Bradford D, Larkins E, Mushti SL, Rodriguez L, Skinner AM, Helms WS, Price LSL, Zirkelbach JF, Li Y, Liu J et al.. (2021)
FDA Approval Summary: Selpercatinib for the Treatment of Lung and Thyroid Cancers with RET Gene Mutations or Fusions. Clin Cancer Res, 27 (8): 2130-2135. [PMID:33239432] |
|
3. Drilon A, Subbiah V, Gautschi O, Tomasini P, de Braud F, Solomon BJ, Shao-Weng Tan D, Alonso G, Wolf J, Park K et al.. (2023)
Selpercatinib in Patients With RET Fusion-Positive Non-Small-Cell Lung Cancer: Updated Safety and Efficacy From the Registrational LIBRETTO-001 Phase I/II Trial. J Clin Oncol, 41 (2): 385-394. [PMID:36122315] |
|
4. Solomon BJ, Zhou CC, Drilon A, Park K, Wolf J, Elamin Y, Davis HM, Soldatenkova V, Sashegyi A, Lin AB et al.. (2021)
Phase III study of selpercatinib versus chemotherapy ± pembrolizumab in untreated RET positive non-small-cell lung cancer. Future Oncol, 17 (7): 763-773. [PMID:33150799] |
|
5. Subbiah V, Velcheti V, Tuch BB, Ebata K, Busaidy NL, Cabanillas ME, Wirth LJ, Stock S, Smith S, Lauriault V et al.. (2018)
Selective RET kinase inhibition for patients with RET-altered cancers. Ann Oncol, 29 (8): 1869-1876. [PMID:29912274] |
|
6. Subbiah V, Wolf J, Konda B, Kang H, Spira A, Weiss J, Takeda M, Ohe Y, Khan S, Ohashi K et al.. (2022)
Tumour-agnostic efficacy and safety of selpercatinib in patients with RET fusion-positive solid tumours other than lung or thyroid tumours (LIBRETTO-001): a phase 1/2, open-label, basket trial. Lancet Oncol, 23 (10): 1261-1273. [PMID:36108661] |
|
7. Tsuboi M, Goldman JW, Wu YL, Johnson ML, Paz-Ares L, Yang JC, Besse B, Su W, Chao BH, Drilon A. (2022)
LIBRETTO-432, a phase III study of adjuvant selpercatinib or placebo in stage IB-IIIA RET fusion-positive non-small-cell lung cancer. Future Oncol, 18 (28): 3133-3141. [PMID:35950566] |
|
8. Wirth LJ, Brose MS, Elisei R, Capdevila J, Hoff AO, Hu MI, Tahara M, Robinson B, Gao M, Xia M et al.. (2022)
LIBRETTO-531: a phase III study of selpercatinib in multikinase inhibitor-naïve RET-mutant medullary thyroid cancer. Future Oncol, 18 (28): 3143-3150. [PMID:35969032] |
|
9. Zheng X, Ji Q, Sun Y, Ge M, Zhang B, Cheng Y, Lei S, Shi F, Guo Y, Li L et al.. (2022)
Efficacy and safety of selpercatinib in Chinese patients with advanced RET-altered thyroid cancers: results from the phase II LIBRETTO-321 study. Ther Adv Med Oncol, 14: 17588359221119318. [PMID:36062046] |
|
10. Zhou C, Solomon B, Loong HH, Park K, Pérol M, Arriola E, Novello S, Han B, Zhou J, Ardizzoni A et al.. (2023)
First-Line Selpercatinib or Chemotherapy and Pembrolizumab in RET Fusion-Positive NSCLC. N Engl J Med, 389 (20): 1839-1850. [PMID:37870973] |
|
11. (2019)
First RET Inhibitor on Path to FDA Approval. Cancer Discov, 9 (11): 1476-1477. DOI: 10.1158/2159-8290.CD-NB2019-112 [PMID:31554640] |