|
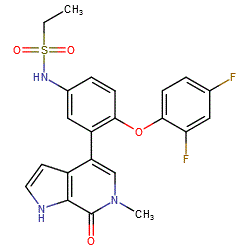
Synonyms: ABBV-075 | ABBV075 | Example 36 [WO2013097601]
Compound class:
Synthetic organic
Comment: Mivebresib (ABBV-075) is a potent orally available BET inhibitor [1]. BET inhibitors bind to the bromodomains of the bromodomain and extra-terminal motif (BET) proteins BRD2, BRD3, BRD4, and BRDT. This mechanism acts to prevent protein-protein interaction between BET proteins and acetylated histones and transcription factors. The action disrupts chromatin remodeling and inhibits expression of some growth-promoting genes which slows proliferation in susceptible tumours.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| A Phase 1 clinical trial evaluating the safety and pharmacokinetics of mivebresib (ABBV-075) in cancer patients (NCT02391480) has been completed. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02391480 | A Study Evaluating the Safety and Pharmacokinetics of ABBV-075 in Subjects With Cancer | Phase 1 Interventional | AbbVie | ||