|
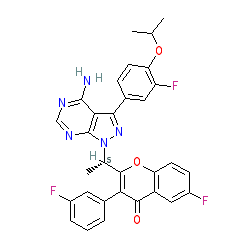
Synonyms: example A1 [US2014011819] | RP-5264 | RP5264 | TGR-1202 | TGR1202 | Ukoniq®
umbralisib is an approved drug (FDA (2021))
Compound class:
Synthetic organic
Comment: Umbralisib (TGR-1202; RP5264,) is an orally available, selective inhibitor of the δ isoform of phosphoinositide 3-kinase (PI3K) [1,3,5], that was developed by TG Therapeutics for the treatment of several B cell malignancies. It also inhibits casein kinase-1ε [2]. For clinical application umbralisib is formulated as the tosylate salt.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Umbralisib was evaluated in a number of clinical trials in patients with various hematological malignancies and successfully progressed to Phase 3 in combination with ublituximab (and in comparison to obinutuzumab + chlorambucil) in CLL (NCT02612311). Phase 1 study results indicated that umbralisib was well tolerated and showed preliminary signs efficacy in patients with relapsed/refractory haematological malignancies, and had an improved safety profile in comparison to other PI3Kδ inhibitors [2]. In January 2019, TG Therapeutics announced that the FDA had granted Breakthrough Therapy Designation for umbralisib for the treatment of adult patients with marginal zone lymphoma (MZL, an indolent B-cell non-Hodgkin lymphoma) who have received at least one prior anti-CD20 therapy [4], based on interim data from the MZL cohort receiving umbralisib monotherapy in the UNITY-NHL trial NCT02793583. Accelerated FDA approval was granted in February 2021, for the MZL indication and for relapsed/refractory follicular lymphoma (FL) in patients who have been treated with >3 systemic therapy regimens. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02612311 | Ublituximab + TGR-1202 Compared to Obinutuzumab + Chlorambucil in Patients With Untreated and Previously Treated Chronic Lymphocytic Leukemia | Phase 3 Interventional | TG Therapeutics, Inc. | ||
| NCT02793583 | Study to Assess the Efficacy and Safety of Ublituximab + TGR-1202 With or Without Bendamustine and TGR-1202 Alone in Patients With Previously Treated Non-Hodgkins Lymphoma | Phase 2/Phase 3 Interventional | TG Therapeutics, Inc. | ||