|
Synonyms: PLX-3397 | PLX3397 | Turalio®
pexidartinib is an approved drug (FDA (2019))
Compound class:
Synthetic organic
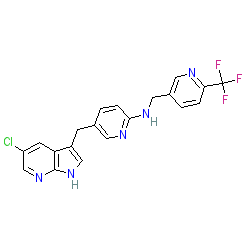
Comment: Pexidartinib is an orally available, multi-targeted inhibitor of the receptor tyrosine kinases, Fms-related tyrosine kinase 3 (FLT3), stem cell growth factor receptor (KIT) and colony stimulating factor 1 receptor (CSF1R), with potential antineoplastic activity [5]. Pexidartinib is compound P-0181 in patent US7893075 B2 [6].
It is active against wild type FLT3 and the FLT3 gatekeeper F691L resistance mutant, but its activity is vulnerable to mutations in the FLT3 activation loop [4]. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| The US FDA granted pexidartinib orphan drug designation for the proliferative disorder tenosynovial giant cell tumour (TGCT; a.k.a. pigmented villonodular synovitis or PVNS) in 2014. Following Priority Review, the FDA approved pexidartinib in August 2019, as a treament for symptomatic TGCT in adult patients with severe morbidity or functional limitations, and whose symptoms are not improved by surgery. |
Mechanism Of Action and Pharmacodynamic Effects  |
| CSF1R is a primary driver in PVNS [1-3], and expression is elevated in most tumours of this type [5]. PLX3397 stabilises the kinase in an autoinhibited conformation, the clinical outcome of which is partial response or stable disease in patients with PVNS [5]. |