|
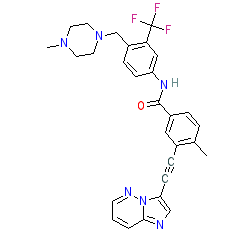
Synonyms: AP24534 | Iclusig®
ponatinib is an approved drug (FDA (2012), EMA (2013))
Compound class:
Synthetic organic
Comment: Ponatinib is a Type-1 kinase inhibitor. It is a third generation BCR-Abl inhibitor. Use of ponatinib is subject to additional monitoring due to the observed serious risk of liver problems or blood clots (including heart attack and stroke, collectively referred to as arterial occlusive events, or AOEs). Final 5-year results of safety and efficacy in Ph+ leukemia as evaluated in NCT01207440 are reported by Cortes et al. (2018) [1].
Marketed formulations contain ponatinib hydrochloride (PubChem CID 46908927). Ponatinib has also been reported as a dual inhibitor of RIPK1 and RIPK3 which inhibits experimental models of RIPK1- and RIPK3-dependent cell death (necroptosis) [5]. On the basis of these findings, hybrid ponatinib/necrostatin-1 (a RIPK1 and IDO inhibitor) were designed and tested for potential to target RIPK1- and RIPK3-driven inflammatory pathologies. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖
View more information in the IUPHAR Pharmacology Education Project: ponatinib |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Ponatinib is a multi-targeted tyrosine-kinase inhibitor approved for the treatment of adult patients with chronic phase, accelerated phase, or blast phase chronic myeloid leukemia (CML) or Philadelphia chromosome positive acute lymphoblastic leukemia (Ph+ALL) that is resistant or intolerant to prior tyrosine kinase inhibitor therapy. For example, cases of CML which have the T315I mutation, are resistant to current therapies such as imatinib so ponatinib has been designed to be effective against these types of tumours. Final 5-year results of safety and efficacy in Ph+ leukemia as evaluated in Phase 2 study NCT01207440 are reported by Cortes et al. (2018) [1]. This analysis concludes that ponatinib is an effective treatment that produces durable and clinically significant responses, but also, that the risk of AOEs should be considered on a patient-by-patient basis to fully inform the decision to treat with ponatinib. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT01207440 | Ponatinib for Chronic Myeloid Leukemia (CML) Evaluation and Ph+ Acute Lymphoblastic Leukemia (ALL) | Phase 2 Interventional | Takeda | ||
External links  |
|
For extended ADME data see the following: Electronic Medicines Compendium (eMC) Drugs.com European Medicines Agency (EMA) |