|
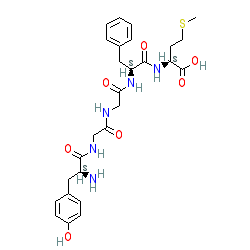
Synonyms: 1-5 Adrenorphin | [Met]-enkephalin | IRT-101 | methionine enkephalin | np2 enkephalin | opioid growth factor (OGF)
Compound class:
Endogenous peptide in human, mouse or rat
Comment: MET-enkephalin (MENK) is an endogenous opioid peptide. It exhibits agonist activity at the δ- and μ-opioid receptors. MENK produces immunomodulatory, neuromodulatory, antinociceptive/analgesic, antidepressant, and gastrointestinal motility modulating activities. It has cytostatic activity vs. cancer cells and may help recruit NK cells to kill tumour cells [3,5,8].
Species: Human, Mouse, Rat
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
| No information available. |
Summary of Clinical Use  |
| The immuno-regulating effects of MENK have been investigated for clinical utility in MS patients, and it has been trialled in cancer patients. EK-12 (Enkorten) is a combination of MENK + tridecactide (alpha-corticotropin 1-13, a.k.a. (Des-acetyl)-α-MSH), that is proposed as a potent immunomodulatory therapy. This is in active clinical trial for relapsing-remitting MS. SARS-CoV-2 infection (COVID-19): EK-12 has also entered clinical trial in COVID-19 patients with moderate-severe disease in Bosnia. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT04374032 | Clinical Trial to Evaluate the Efficacy and Safety of an Immunomodulatory Therapy for the Treatment of Patients With Moderate to Severe COVID-19 Infection | Phase 2/Phase 3 Interventional | Bosnalijek D.D | ||
| NCT03283397 | A Phase IIIb, Multicenter, International Study to Evaluate the Efficacy, Safety and Tolerability of EK-12 in Patients With RRMS | Phase 3 Interventional | Bosnalijek D.D | ||