|
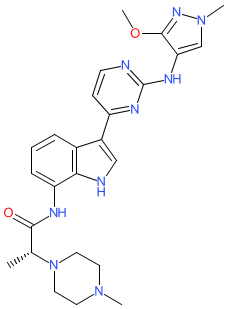
Synonyms: AZD-4205 | AZD4205 | compound 21 [PMID: 32297743] | example 32 [US9714236B2] [1] | JAK1-IN-3
Compound class:
Synthetic organic
Comment: AZD4205 is a selective, orally bioavailable, ATP-competitive inhibitor of Janus kinase 1 (JAK1) that was developed by Dizal Pharmaceutical Company [2]. It has been shown to enhance antitumour activity in combination with osimertinib (an approved EGFR inhibitor) in a preclinical non-small cell lung cancer xenograft model. A higher drug concentration within the gastrointestinal tract relative to plasma in preclinical models suggests potential to treat inflammatory bowel disease. Persistently activated STAT3 has been shown to be oncogenic, and drives expression of cellular proteins which contribute to central processes in cancer progression (survival, proliferation, invasion, angiogenesis). JAK1 is the key Janus kinase responsible for cytokine-mediated STAT3 activation.
The chemical structure of AZD4205 matches that for the INN golidocitinib which was surfaced in WHO Proposed list 125 in July 2021. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT03450330 | Assessing an Oral Janus Kinase Inhibitor, AZD4205, in Combination With Osimertinib in Patients Who Have Advanced Non-small Cell Lung Cancer | Phase 1/Phase 2 Interventional | Dizal (Jiangsu) Pharmaceutical Co., Ltd. | ||
| NCT04105010 | Assessing An Oral Janus Kinase Inhibitor, AZD4205 as Monotherapy in Patients Who Have PTCL (JACKPOT8) | Phase 1/Phase 2 Interventional | Dizal (Jiangsu) Pharmaceutical Co., Ltd. | ||
| NCT03728023 | A Study of AZD4205 in Healthy Adult Subjects | Phase 1 Interventional | Dizal (Jiangsu) Pharmaceutical Co., Ltd. | ||