|
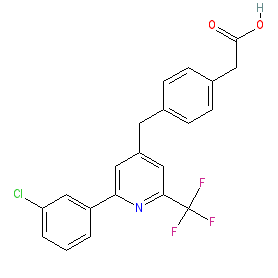
Synonyms: BPN-14770 | compound 28 [PMID: 31013090]
Compound class:
Synthetic organic
Comment: BPN14770 is reported as a selective allosteric inhibitor of phosphodiesterase 4D (PDE4D) [3]. BPN14770 achieves isotype selectivity by binding to a single amino acid difference on the UCR2 regulatory domain of PDE4D that controls access of cAMP substrate to the enzyme's catalytic site. Dimeric isoforms of PDE4D appear to be important for normal brain function [2], with abnormally/inappropriately active isoform dimers being associated with intellectual disability [1].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||