|
Synonyms: GSK-1160724 | GSK1160724 | TD4208 | Yupelri®
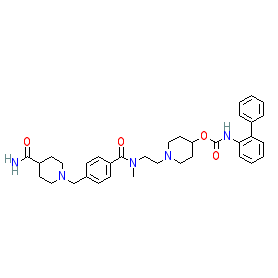
revefenacin is an approved drug (FDA (2018))
Compound class:
Synthetic organic
Comment: Revefenacin (TD-4208) is a potent and long-acting muscarinic cholinergic receptor (mAChR) antagonist (LAMA) that has bronchodilatory effects [1-2], and which is approved for the management of chronic obstructive pulmonary disease (COPD). mAChR antagonists are the core agents used as first-line therapy for COPD. Revefenacin acts as a competitive antagonist at all 5 human mAChRs [1].
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| Revefenacin was FDA approved for the maintenance treatment of patients with COPD in November 2018. It is a once-daily therapeutic that is delivered as a nebulized inhalation solution. Phase 2 clinical trial results were published by Quinn et al. in 2018 [3]. |