|
Synonyms: compound 44 [PMID: 30249354]
Compound class:
Synthetic organic
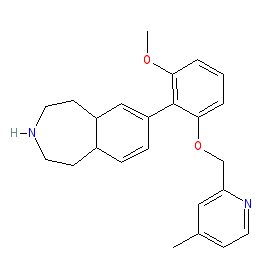
Comment: GSK2646264 is a skin permeable Syk inhibitor that is being investigated as a topical treatment for skin inflammation [1]. An X-ray crystal structure of GSK2646264 bound to human SYK reveals that it binds the enzyme's aspartate 512 residue which lies within the ribose pocket in the ATP binding site of Syk.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| No information available. |
Summary of Clinical Use  |
| GSK2646264 has completed Phase 1 clinical evaluation in patients with urticaria (NCT02424799) and cutaneous lupus erythematosus (NCT02927457). |
| Clinical Trials | |||||
| Clinical Trial ID | Title | Type | Source | Comment | References |
| NCT02424799 | Study to Investigate Safety, Tolerability, Pharmacodynamics and Pharmacokinetics of GSK2646264 | Phase 1 Interventional | GlaxoSmithKline | ||
| NCT02927457 | Safety, Tolerability, Pharmacokinetics, Pharmacodynamics and Clinical Effect of GSK2646264 in Cutaneous Lupus Erythematosus Subjects | Phase 1 Interventional | GlaxoSmithKline | ||