|
Synonyms: Antepsin® | Carafate®
sucralfate is an approved drug (FDA (1981))
Compound class:
Synthetic organic
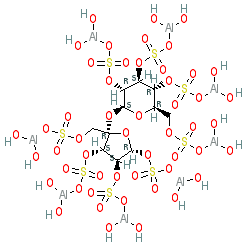
Comment: Sucralfate is a complex of sucrose, sulfate and aluminum which is basic in nature and has an overall negative charge.
Note that this is a complicated chemical complex. Different chemical databases represent this compound in different ways, all of which are valid. The ChEMBL compound is represented by the entry CHEMBL2367706, with separate aluminium hydroxide ions, whereas the structure we show has these ions in association with the sulphites on the core disaccharide sugar. PubChem CID 70789197 also represents the aluminium hydroxide groups in a different way. 
View more information in the IUPHAR Pharmacology Education Project: sucralfate |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| This drug attaches non-specifically to proteins in ulcerated tissue (such as albumin and fibrinogen). Acid-induced cross-linking creates a viscous, paste-like material which acts as a protective barrier. This drug therefore has no 'primary' molecular target. |