|
Synonyms: Bilessglu® | CS-038 | CS038
chiglitazar is an approved drug (China (2022))
Compound class:
Synthetic organic
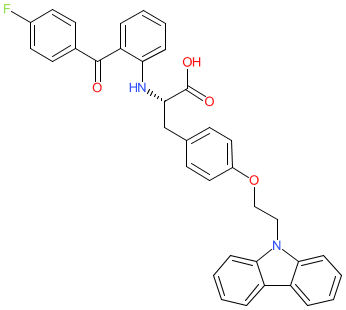
Comment: Chiglitazar (CS-038) is a synthetic small-molecule pan-agonist of peroxisome proliferator-activated receptors (PPARs) α, δ and γ [2-3]. Structurally this is the S-enantiomer of carfloglitazar (the INN represents the racemic mixture; PubChem CID 9938010). Chiglitazar upregulates the expression of genes downstream of PPARα/δthat are involved in lipid metabolism and thermogenesis. It was developed for potential to treat type 2 diabetes (T2D) and non-alcoholic steatohepatitis (NASH).
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| Preclinically chiglitazar improved impaired insulin and glucose tolerance in monosodium L-glutamate obese rats [3]. |
| Selectivity at nuclear hormone receptors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||