|
Compound class:
Synthetic organic
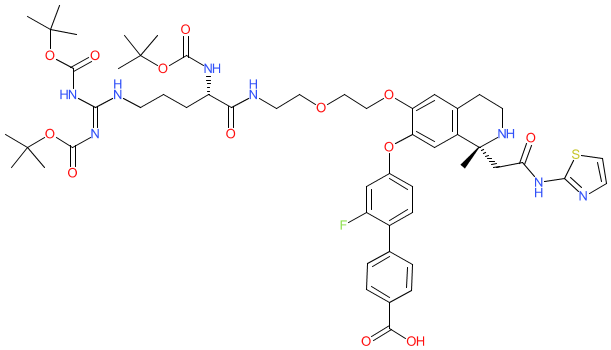
Comment: Compound 16 is a hybrid, hetero-bifunctional molecule that comprises a PCSK9-binding ligand connected to an Arg(Boc)3 moiety [3]. This latter motif acts as a ubiquitin-independent proteasome-recruiting element [1-2,4] that in this case was used to facilitate ligand-induced degradation of PCSK9 protein. X-ray co-crystallisation shows that compound 16 binds to an allosteric site on PCSK9 and has no intrinsic effect on PCSK9 function. The X-ray structure also reveals that the Arg(Boc)3 moiety extents outwith the binding pocket which enables interactions with components of the degradation complex. Targeted degradation of PCSK9 is being examined as an alternative to anti-PCSK9 monoclonals (already in the clinic) or small-molecule inhibitors of the PCSK9/low-density lipoprotein (LDL) receptor protein-protein interaction (which is very difficult to drug due to the 3D structure of the interaction domains) as a novel mechanism to decrease LDL cholesterol levels.
The Arg(Boc)3 motif is used as an alternative to the more widely utilised ubiquitin-proteasome system recruiting elements that engage the VHL and CRBN ubiquitin ligases, and which have been used in a number of previously reported PROTACs. Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| In HEK293 cells overexpressing PCSK9, 20 μM compound 16 achieves a maximum degradation of pro and mature forms of PCSK9 of 58% and 61%, respectively [3]. It does not appear to be cytotoxic in this system. |
| Selectivity at enzymes | ||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||