|
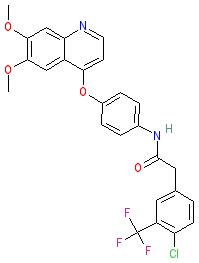
Synonyms: compound 18 [PMID: 31250638]
Compound class:
Synthetic organic
Comment: CHMFL-KIT-64 is a potent and orally available inhibitor of wild-type c-KIT and a range of known c-KIT resistance mutants [1]. It has demonstrated anti-tumour efficacy in vitro, in mouse xenograft models, and against imatinib-resistant patient tumour cells expressing wild-type c-KIT. It has been proposed as a potential clinical lead for gastrointestinal stromal tumour (GIST) therapy as the majority (80-85%) of GISTs harbour c-KIT activation mutations. Other c-KIT mutations confer primary or secondary (acquired) resistance to imatinib which is the first line drug used to combat GISTs. CHMFL-KIT-64 was designed to target mutations that are refractory to currently approved therapeutics including imatinib, sunitinib and regorafenib. It can potently inhibit the T670I ATP binding pocket (gatekeeper) mutant that is resistant to many other c-KIT inhibitors.
Ligand Activity Visualisation ChartsThese are box plot that provide a unique visualisation, summarising all the activity data for a ligand taken from ChEMBL and GtoPdb across multiple targets and species. Click on a plot to see the median, interquartile range, low and high data points. A value of zero indicates that no data are available. A separate chart is created for each target, and where possible the algorithm tries to merge ChEMBL and GtoPdb targets by matching them on name and UniProt accession, for each available species. However, please note that inconsistency in naming of targets may lead to data for the same target being reported across multiple charts. ✖ |
|
|||||||||||||||||||||||||||||||||||
| Bioactivity Comments |
| CHMFL-KIT-64 exhibits favourable potencies against the majority of the gain-of-function mutations in the juxtamembrane domain, drug-resistant mutations in the ATP binding pocket (but not V654A), and activation loops (except D816V). CHMFL-KIT-64 inhibits proliferation of BaF3 cells expressing the c-KIT T670I mutant by 90%, and inhibits enzyme activity with an IC50 of 8 nM in a biochemical assay [1]. Kinome screening reveals that c-KIT, PDGFRα, PDGFRβ, and CSF1R kinases are the main targets of CHMFL-KIT-64. These are all type III receptor tyrosine kinase family members whose ATP binding pockets are highly conserved. |
| Selectivity at catalytic receptors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||