mechanistic target of rapamycin kinase
Target id: 2109
Nomenclature: mechanistic target of rapamycin kinase
Abbreviated Name: mTOR
Family: FRAP subfamily
Contents:
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | - | 2549 | 1p36.22 | MTOR | mechanistic target of rapamycin kinase | |
| Mouse | - | 2549 | 4 78.76 cM | Mtor | mechanistic target of rapamycin kinase | |
| Rat | - | 2549 | 5q36 | Mtor | mechanistic target of rapamycin kinase | |
Database Links  |
|
| Alphafold | P42345 (Hs), Q9JLN9 (Mm), P42346 (Rn) |
| BRENDA | 2.7.11.1 |
| CATH/Gene3D | 1.10.1070.11, 1.20.120.150, 1.25.10.10 |
| ChEMBL Target | CHEMBL2842 (Hs), CHEMBL1255165 (Mm), CHEMBL1075134 (Rn) |
| DrugBank Target | P42345 (Hs) |
| Ensembl Gene | ENSG00000198793 (Hs), ENSMUSG00000028991 (Mm), ENSRNOG00000009615 (Rn) |
| Entrez Gene | 2475 (Hs), 56717 (Mm), 56718 (Rn) |
| Human Protein Atlas | ENSG00000198793 (Hs) |
| KEGG Enzyme | 2.7.11.1 |
| KEGG Gene | hsa:2475 (Hs), mmu:56717 (Mm), rno:56718 (Rn) |
| OMIM | 601231 (Hs) |
| Pharos | P42345 (Hs) |
| RefSeq Nucleotide | NM_004958 (Hs), NM_020009 (Mm), NM_019906 (Rn) |
| RefSeq Protein | NP_004949 (Hs), NP_064393 (Mm), NP_063971 (Rn) |
| SynPHARM |
82830 (in complex with compound 82 [PMID: 21332118]) 80974 (in complex with PI-103) 81767 (in complex with PP-242) 82891 (in complex with torin 2) |
| UniProtKB | P42345 (Hs), Q9JLN9 (Mm), P42346 (Rn) |
| Wikipedia | MTOR (Hs) |
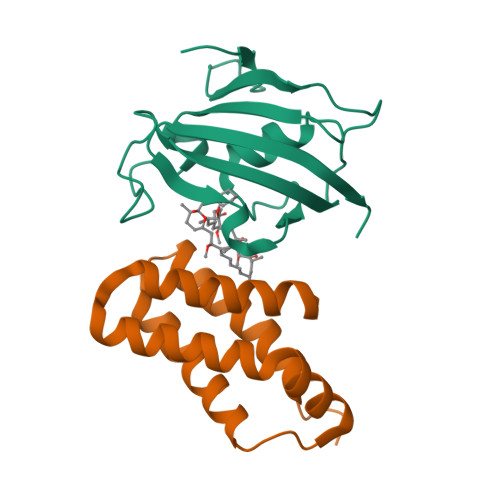
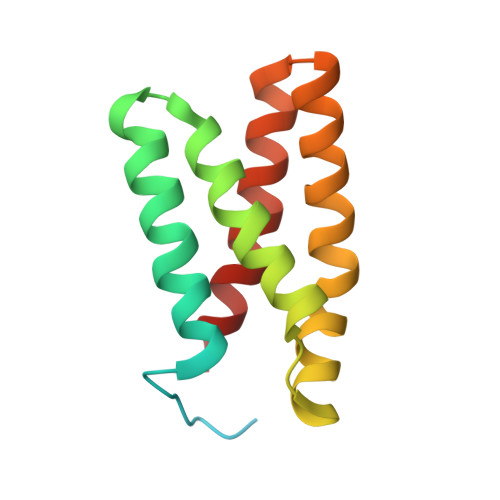
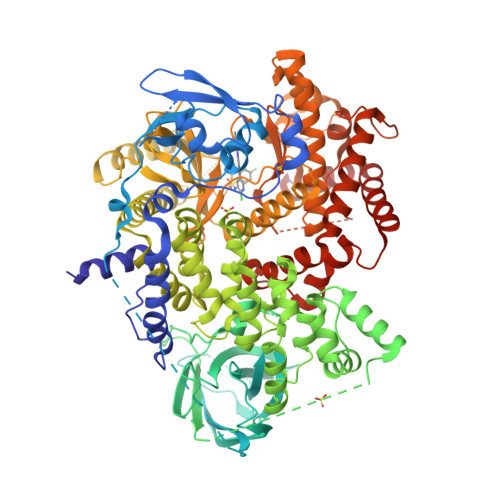
Selected 3D Structures  |
|||||||||||||
|
|
||||||||||||
|
|
||||||||||||
|
|
||||||||||||
Enzyme Reaction  |
||||
|
||||
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DiscoveRx KINOMEscan® screen  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen of 72 inhibitors against 456 human kinases. Quantitative data were derived using DiscoveRx KINOMEscan® platform. http://www.discoverx.com/services/drug-discovery-development-services/kinase-profiling/kinomescan Reference: 9,42 |
 
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: MTOR | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EMD Millipore KinaseProfilerTM screen/Reaction Biology Kinase HotspotSM screen  |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen profiling 158 kinase inhibitors (Calbiochem Protein Kinase Inhibitor Library I and II, catalogue numbers 539744 and 539745) for their inhibitory activity at 1µM and 10µM against 234 human recombinant kinases using the EMD Millipore KinaseProfilerTM service. A screen profiling the inhibitory activity of 178 commercially available kinase inhibitors at 0.5µM against a panel of 300 recombinant protein kinases using the Reaction Biology Corporation Kinase HotspotSM platform. http://www.millipore.com/techpublications/tech1/pf3036 http://www.reactionbiology.com/webapps/main/pages/kinase.aspx Reference: 12... |

|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: mTOR-FKBP12/nd | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: mTOR/nd | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Immuno Process Associations | ||
|
||
|
||
|
||
|
||
|
||
|
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||||||||||
|
||||||||||||||||||
| Clinically-Relevant Mutations and Pathophysiology Comments | ||||||||||||||||||
| Hyperactivation of mTOR kinase by single amino acid mutations such as M2327I (which has been identified in drug-naive patients) can reduce sensitivity to ATP-competitive mTOR inhibitors in vitro [36]. | ||||||||||||||||||
References
1. Apsel B, Blair JA, Gonzalez B, Nazif TM, Feldman ME, Aizenstein B, Hoffman R, Williams RL, Shokat KM, Knight ZA. (2008) Targeted polypharmacology: discovery of dual inhibitors of tyrosine and phosphoinositide kinases. Nat Chem Biol, 4 (11): 691-9. [PMID:18849971]
2. Barda DA, Mader MM. (2013) PI3 kinase/mTOR dual inhibitor. Patent number: US8440829 B2. Assignee: Eli Lilly And Company. Priority date: 14/01/2011. Publication date: 14/05/2013.
3. Bonazzi S, Goold CP, Gray A, Thomsen NM, Nunez J, Karki RG, Gorde A, Biag JD, Malik HA, Sun Y et al.. (2020) Discovery of a Brain-Penetrant ATP-Competitive Inhibitor of the Mechanistic Target of Rapamycin (mTOR) for CNS Disorders. J Med Chem, 63 (3): 1068-1083. [PMID:31955578]
4. Borsari C, Rageot D, Dall'Asen A, Bohnacker T, Melone A, Sele AM, Jackson E, Langlois JB, Beaufils F, Hebeisen P et al.. (2019) A Conformational Restriction Strategy for the Identification of a Highly Selective Pyrimido-pyrrolo-oxazine mTOR Inhibitor. J Med Chem, 62 (18): 8609-8630. [PMID:31465220]
5. Brown SD, Matthews DJ. (2012) (alpha- substituted aralkylamino and heteroarylalkylamino) pyrimidinyl and 1,3,5 -triazinyl benzimidazoles, pharmaceutical compositions containing them, and these compounds for use in treating proliferative diseases. Patent number: WO2012135160A1. Assignee: Pathway Therapeutics Inc.. Priority date: 28/03/2011. Publication date: 04/10/2012.
6. Cansfield AD, Ladduwahetty T, Sunose M, Ellard K, Lynch R, Newton AL, Lewis A, Bennett G, Zinn N, Thomson DW et al.. (2016) CZ415, a Highly Selective mTOR Inhibitor Showing in Vivo Efficacy in a Collagen Induced Arthritis Model. ACS Med Chem Lett, 7 (8): 768-73. [PMID:27563401]
7. Castro-Falcón G, Seiler GS, Demir Ö, Rathinaswamy MK, Hamelin D, Hoffmann RM, Makowski SL, Letzel AC, Field SJ, Burke JE et al.. (2018) Neolymphostin A Is a Covalent Phosphoinositide 3-Kinase (PI3K)/Mammalian Target of Rapamycin (mTOR) Dual Inhibitor That Employs an Unusual Electrophilic Vinylogous Ester. J Med Chem, 61 (23): 10463-10472. [PMID:30380865]
8. D'Angelo ND, Kim TS, Andrews K, Booker SK, Caenepeel S, Chen K, D'Amico D, Freeman D, Jiang J, Liu L et al.. (2011) Discovery and optimization of a series of benzothiazole phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) dual inhibitors. J Med Chem, 54 (6): 1789-811. [PMID:21332118]
9. Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. (2011) Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1046-51. [PMID:22037378]
10. Fokas E, Prevo R, Pollard JR, Reaper PM, Charlton PA, Cornelissen B, Vallis KA, Hammond EM, Olcina MM, Gillies McKenna W et al.. (2012) Targeting ATR in vivo using the novel inhibitor VE-822 results in selective sensitization of pancreatic tumors to radiation. Cell Death Dis, 3: e441. [PMID:23222511]
11. Fraser C, Carragher NO, Unciti-Broceta A. (2016) eCF309: a potent, selective and cell-permeable mTOR inhibitor. Medchemcomm, 7 (3): 471-477.
12. Gao Y, Davies SP, Augustin M, Woodward A, Patel UA, Kovelman R, Harvey KJ. (2013) A broad activity screen in support of a chemogenomic map for kinase signalling research and drug discovery. Biochem J, 451 (2): 313-28. [PMID:23398362]
13. García-Martínez JM, Moran J, Clarke RG, Gray A, Cosulich SC, Chresta CM, Alessi DR. (2009) Ku-0063794 is a specific inhibitor of the mammalian target of rapamycin (mTOR). Biochem J, 421 (1): 29-42. [PMID:19402821]
14. Gopalsamy A, Bennett EM, Shi M, Zhang WG, Bard J, Yu K. (2012) Identification of pyrimidine derivatives as hSMG-1 inhibitors. Bioorg Med Chem Lett, 22 (21): 6636-41. [PMID:23021994]
15. Hart S, Novotny-Diermayr V, Goh KC, Williams M, Tan YC, Ong LC, Cheong A, Ng BK, Amalini C, Madan B et al.. (2013) VS-5584, a novel and highly selective PI3K/mTOR kinase inhibitor for the treatment of cancer. Mol Cancer Ther, 12 (2): 151-61. [PMID:23270925]
16. Heffron TP, Ndubaku CO, Salphati L, Alicke B, Cheong J, Drobnick J, Edgar K, Gould SE, Lee LB, Lesnick JD et al.. (2016) Discovery of Clinical Development Candidate GDC-0084, a Brain Penetrant Inhibitor of PI3K and mTOR. ACS Med Chem Lett, 7 (4): 351-6. [PMID:27096040]
17. Hsieh AC, Liu Y, Edlind MP, Ingolia NT, Janes MR, Sher A, Shi EY, Stumpf CR, Christensen C, Bonham MJ et al.. (2012) The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature, 485 (7396): 55-61. [PMID:22367541]
18. Jalota-Badhwar A, Bhatia DR, Boreddy S, Joshi A, Venkatraman M, Desai N, Chaudhari S, Bose J, Kolla LS, Deore V et al.. (2015) P7170: A Novel Molecule with Unique Profile of mTORC1/C2 and Activin Receptor-like Kinase 1 Inhibition Leading to Antitumor and Antiangiogenic Activity. Mol Cancer Ther, 14 (5): 1095-106. [PMID:25700704]
19. Kashiyama T, Oda K, Ikeda Y, Shiose Y, Hirota Y, Inaba K, Makii C, Kurikawa R, Miyasaka A, Koso T et al.. (2014) Antitumor activity and induction of TP53-dependent apoptosis toward ovarian clear cell adenocarcinoma by the dual PI3K/mTOR inhibitor DS-7423. PLoS One, 9 (2): e87220. [PMID:24504419]
20. Knight SD, Adams ND, Burgess JL, Chaudhari AM, Darcy MG, Donatelli CA, Luengo JI, Newlander KA, Parrish CA, Ridgers LH et al.. (2010) Discovery of GSK2126458, a Highly Potent Inhibitor of PI3K and the Mammalian Target of Rapamycin. ACS Med Chem Lett, 1 (1): 39-43. [PMID:24900173]
21. Kong F, Zhu T, Yu K, Pagano TG, Desai P, Radebaugh G, Fawzi M. (2011) Isolation and structure of homotemsirolimuses A, B, and C. J Nat Prod, 74 (4): 547-53. [PMID:21438579]
22. Liu F, Wang J, Yang X, Li B, Wu H, Qi S, Chen C, Liu X, Yu K, Wang W et al.. (2016) Discovery of a Highly Selective STK16 Kinase Inhibitor. ACS Chem Biol, 11 (6): 1537-43. [PMID:27082499]
23. Liu KK, Zhu J, Smith GL, Yin MJ, Bailey S, Chen JH, Hu Q, Huang Q, Li C, Li QJ et al.. (2011) Highly Selective and Potent Thiophenes as PI3K Inhibitors with Oral Antitumor Activity. ACS Med Chem Lett, 2 (11): 809-13. [PMID:24900269]
24. Liu N, Rowley BR, Bull CO, Schneider C, Haegebarth A, Schatz CA, Fracasso PR, Wilkie DP, Hentemann M, Wilhelm SM et al.. (2013) BAY 80-6946 is a highly selective intravenous PI3K inhibitor with potent p110α and p110δ activities in tumor cell lines and xenograft models. Mol Cancer Ther, 12 (11): 2319-30. [PMID:24170767]
25. Liu Q, Chang JW, Wang J, Kang SA, Thoreen CC, Markhard A, Hur W, Zhang J, Sim T, Sabatini DM et al.. (2010) Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benzo[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem, 53 (19): 7146-55. [PMID:20860370]
26. Liu Q, Wang J, Kang SA, Thoreen CC, Hur W, Ahmed T, Sabatini DM, Gray NS. (2011) Discovery of 9-(6-aminopyridin-3-yl)-1-(3-(trifluoromethyl)phenyl)benzo[h][1,6]naphthyridin-2(1H)-one (Torin2) as a potent, selective, and orally available mammalian target of rapamycin (mTOR) inhibitor for treatment of cancer. J Med Chem, 54 (5): 1473-80. [PMID:21322566]
27. Maira SM, Stauffer F, Brueggen J, Furet P, Schnell C, Fritsch C, Brachmann S, Chène P, De Pover A, Schoemaker K et al.. (2008) Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther, 7 (7): 1851-63. [PMID:18606717]
28. Mortensen DS, Perrin-Ninkovic SM, Shevlin G, Zhao J, Packard G, Bahmanyar S, Correa M, Elsner J, Harris R, Lee BG et al.. (2015) Discovery of mammalian target of rapamycin (mTOR) kinase inhibitor CC-223. J Med Chem, 58 (13): 5323-33. [PMID:26083478]
29. März AM, Fabian AK, Kozany C, Bracher A, Hausch F. (2013) Large FK506-Binding Proteins Shape the Pharmacology of Rapamycin. Mol Cell Biol, 33 (7): 1357-67. [PMID:23358420]
30. Ohwada J, Ebiike H, Kawada H, Tsukazaki M, Nakamura M, Miyazaki T, Morikami K, Yoshinari K, Yoshida M, Kondoh O et al.. (2011) Discovery and biological activity of a novel class I PI3K inhibitor, CH5132799. Bioorg Med Chem Lett, 21 (6): 1767-72. [PMID:21316229]
31. Pike KG, Malagu K, Hummersone MG, Menear KA, Duggan HM, Gomez S, Martin NM, Ruston L, Pass SL, Pass M. (2013) Optimization of potent and selective dual mTORC1 and mTORC2 inhibitors: the discovery of AZD8055 and AZD2014. Bioorg Med Chem Lett, 23 (5): 1212-6. [PMID:23375793]
32. Rageot D, Bohnacker T, Melone A, Langlois JB, Borsari C, Hillmann P, Sele AM, Beaufils F, Zvelebil M, Hebeisen P et al.. (2018) Discovery and Preclinical Characterization of 5-[4,6-Bis({3-oxa-8-azabicyclo[3.2.1]octan-8-yl})-1,3,5-triazin-2-yl]-4-(difluoromethyl)pyridin-2-amine (PQR620), a Highly Potent and Selective mTORC1/2 Inhibitor for Cancer and Neurological Disorders. J Med Chem, 61 (22): 10084-10105. [PMID:30359003]
33. Raynaud FI, Eccles SA, Patel S, Alix S, Box G, Chuckowree I, Folkes A, Gowan S, De Haven Brandon A, Di Stefano F et al.. (2009) Biological properties of potent inhibitors of class I phosphatidylinositide 3-kinases: from PI-103 through PI-540, PI-620 to the oral agent GDC-0941. Mol Cancer Ther, 8 (7): 1725-38. [PMID:19584227]
34. Ren P, Liu Y, Li L, Chan K, Wilson TE, Campbell SF. (2013) Heterocyclic compounds and uses thereof. Patent number: US20130035324 A1. Assignee: Ren P, Liu Y, Li L, Chan K, Wilson TE, Campbell SF.. Priority date: 17/08/2009. Publication date: 07/02/2013.
35. Rivera VM, Squillace RM, Miller D, Berk L, Wardwell SD, Ning Y, Pollock R, Narasimhan NI, Iuliucci JD, Wang F et al.. (2011) Ridaforolimus (AP23573; MK-8669), a potent mTOR inhibitor, has broad antitumor activity and can be optimally administered using intermittent dosing regimens. Mol Cancer Ther, 10 (6): 1059-71. [PMID:21482695]
36. Rodrik-Outmezguine VS, Okaniwa M, Yao Z, Novotny CJ, McWhirter C, Banaji A, Won H, Wong W, Berger M, de Stanchina E et al.. (2016) Overcoming mTOR resistance mutations with a new-generation mTOR inhibitor. Nature, 534 (7606): 272-6. [PMID:27279227]
37. Sedrani R, Cottens S, Kallen J, Schuler W. (1998) Chemical modification of rapamycin: the discovery of SDZ RAD. Transplant Proc, 30 (5): 2192-4. [PMID:9723437]
38. Sutherlin DP, Bao L, Berry M, Castanedo G, Chuckowree I, Dotson J, Folks A, Friedman L, Goldsmith R, Gunzner J et al.. (2011) Discovery of a potent, selective, and orally available class I phosphatidylinositol 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) kinase inhibitor (GDC-0980) for the treatment of cancer. J Med Chem, 54 (21): 7579-87. [PMID:21981714]
39. Takeuchi CS, Kim BG, Blazey CM, Ma S, Johnson HW, Anand NK, Arcalas A, Baik TG, Buhr CA, Cannoy J et al.. (2013) Discovery of a novel class of highly potent, selective, ATP-competitive, and orally bioavailable inhibitors of the mammalian target of rapamycin (mTOR). J Med Chem, 56 (6): 2218-34. [PMID:23394126]
40. Venkatesan AM, Dehnhardt CM, Delos Santos E, Chen Z, Dos Santos O, Ayral-Kaloustian S, Khafizova G, Brooijmans N, Mallon R, Hollander I et al.. (2010) Bis(morpholino-1,3,5-triazine) derivatives: potent adenosine 5'-triphosphate competitive phosphatidylinositol-3-kinase/mammalian target of rapamycin inhibitors: discovery of compound 26 (PKI-587), a highly efficacious dual inhibitor. J Med Chem, 53 (6): 2636-45. [PMID:20166697]
41. Veverka V, Crabbe T, Bird I, Lennie G, Muskett FW, Taylor RJ, Carr MD. (2008) Structural characterization of the interaction of mTOR with phosphatidic acid and a novel class of inhibitor: compelling evidence for a central role of the FRB domain in small molecule-mediated regulation of mTOR. Oncogene, 27 (5): 585-95. [PMID:17684489]
42. Wodicka LM, Ciceri P, Davis MI, Hunt JP, Floyd M, Salerno S, Hua XH, Ford JM, Armstrong RC, Zarrinkar PP et al.. (2010) Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry. Chem Biol, 17 (11): 1241-9. [PMID:21095574]
43. Yu K, Toral-Barza L, Shi C, Zhang WG, Lucas J, Shor B, Kim J, Verheijen J, Curran K, Malwitz DJ et al.. (2009) Biochemical, cellular, and in vivo activity of novel ATP-competitive and selective inhibitors of the mammalian target of rapamycin. Cancer Res, 69 (15): 6232-40. [PMID:19584280]
44. Yu Y, Han Y, Zhang F, Gao Z, Zhu T, Dong S, Ma M. (2020) Design, Synthesis, and Biological Evaluation of Imidazo[1,2-a]pyridine Derivatives as Novel PI3K/mTOR Dual Inhibitors. J Med Chem, 63 (6): 3028-3046. [PMID:32069401]
How to cite this page
FRAP subfamily: mechanistic target of rapamycin kinase. Last modified on 19/04/2023. Accessed on 18/04/2024. IUPHAR/BPS Guide to PHARMACOLOGY, https://www.guidetomalariapharmacology.org/GRAC/ObjectDisplayForward?objectId=2109.













