neurotrophic receptor tyrosine kinase 3
Target id: 1819
Nomenclature: neurotrophic receptor tyrosine kinase 3
Abbreviated Name: trkC
Contents:
Gene and Protein Information  |
||||||
| Species | TM | AA | Chromosomal Location | Gene Symbol | Gene Name | Reference |
| Human | 1 | 839 | 15q25.3 | NTRK3 | neurotrophic receptor tyrosine kinase 3 | |
| Mouse | 1 | 825 | 7 44.01 cM | Ntrk3 | neurotrophic tyrosine kinase, receptor, type 3 | |
| Rat | 1 | 864 | 1q31 | Ntrk3 | neurotrophic receptor tyrosine kinase 3 | |
Previous and Unofficial Names  |
| GP145-TrkC | NT-3 growth factor receptor | neural receptor protein-tyrosine kinase (trkC) | neurotrophic tyrosine kinase |
Database Links  |
|
| Alphafold | Q16288 (Hs), Q6VNS1 (Mm), Q03351 (Rn) |
| BRENDA | 2.7.10.1 |
| CATH/Gene3D | 3.80.10.10, 2.60.40.10 |
| ChEMBL Target | CHEMBL5608 (Hs), CHEMBL2791 (Mm) |
| Ensembl Gene | ENSG00000140538 (Hs), ENSMUSG00000059146 (Mm), ENSRNOG00000018674 (Rn) |
| Entrez Gene | 4916 (Hs), 18213 (Mm), 29613 (Rn) |
| Human Protein Atlas | ENSG00000140538 (Hs) |
| KEGG Enzyme | 2.7.10.1 |
| KEGG Gene | hsa:4916 (Hs), mmu:18213 (Mm), rno:29613 (Rn) |
| OMIM | 191316 (Hs) |
| Orphanet | ORPHA246681 (Hs) |
| Pharos | Q16288 (Hs) |
| RefSeq Nucleotide | NM_001007156 (Hs), NM_008746 (Mm), NM_001270656 (Rn) |
| RefSeq Protein | NP_001007157 (Hs), NP_877961 (Mm), NP_032772 (Mm), NP_001257585 (Rn) |
| UniProtKB | Q16288 (Hs), Q6VNS1 (Mm), Q03351 (Rn) |
| Wikipedia | NTRK3 (Hs) |
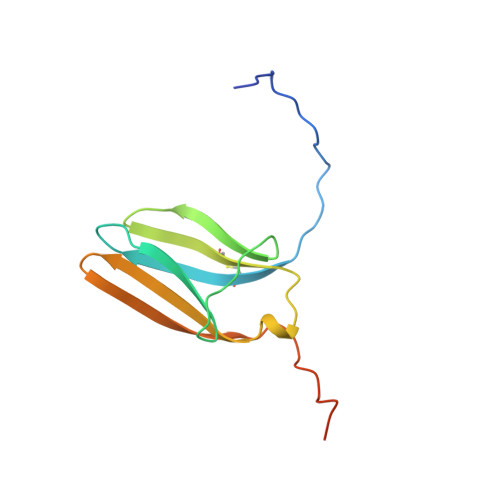
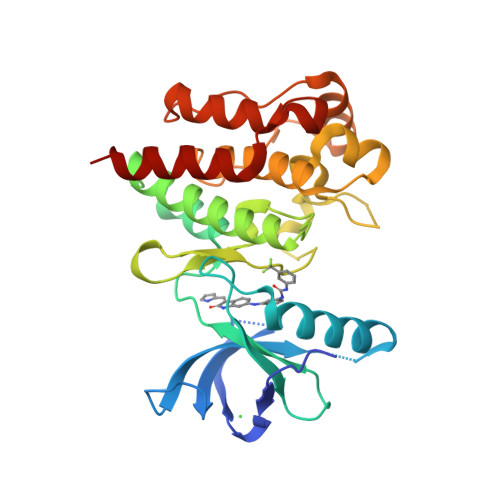
Selected 3D Structures  |
|||||||||||
|
|
||||||||||
|
|
||||||||||
Enzyme Reaction  |
||||
|
||||
Natural/Endogenous Ligands  |
| neurotrophin-3 {Sp: Human} |
Download all structure-activity data for this target as a CSV file 
| Inhibitors | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | View all chemical structures | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| View species-specific inhibitor tables | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
DiscoveRx KINOMEscan® screen  |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen of 72 inhibitors against 456 human kinases. Quantitative data were derived using DiscoveRx KINOMEscan® platform. http://www.discoverx.com/services/drug-discovery-development-services/kinase-profiling/kinomescan Reference: 4,16 |
 
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: TRKC | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
EMD Millipore KinaseProfilerTM screen/Reaction Biology Kinase HotspotSM screen  |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
A screen profiling 158 kinase inhibitors (Calbiochem Protein Kinase Inhibitor Library I and II, catalogue numbers 539744 and 539745) for their inhibitory activity at 1µM and 10µM against 234 human recombinant kinases using the EMD Millipore KinaseProfilerTM service. A screen profiling the inhibitory activity of 178 commercially available kinase inhibitors at 0.5µM against a panel of 300 recombinant protein kinases using the Reaction Biology Corporation Kinase HotspotSM platform. http://www.millipore.com/techpublications/tech1/pf3036 http://www.reactionbiology.com/webapps/main/pages/kinase.aspx Reference: ...2 |

|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Key to terms and symbols | Click column headers to sort | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Target used in screen: nd/TRKC | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displaying the top 10 most potent ligands View all ligands in screen » | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clinically-Relevant Mutations and Pathophysiology 
|
||||||||||
|
||||||||||
|
||||||||||
References
1. Albaugh P, Fan Y, Mi Y, Sun F, Adrian F, Li N, Jia Y, Sarkisova Y, Kreusch A, Hood T et al.. (2012) Discovery of GNF-5837, a Selective TRK Inhibitor with Efficacy in Rodent Cancer Tumor Models. ACS Med Chem Lett, 3 (2): 140-5. [PMID:24900443]
2. Anastassiadis T, Deacon SW, Devarajan K, Ma H, Peterson JR. (2011) Comprehensive assay of kinase catalytic activity reveals features of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1039-45. [PMID:22037377]
3. AstraZeneca. AZD1332. Accessed on 11/09/2014. Modified on 11/09/2014. AstraZeneca.com, http://openinnovation.astrazeneca.com/what-we-offer/compound/azd1332/
4. Davis MI, Hunt JP, Herrgard S, Ciceri P, Wodicka LM, Pallares G, Hocker M, Treiber DK, Zarrinkar PP. (2011) Comprehensive analysis of kinase inhibitor selectivity. Nat Biotechnol, 29 (11): 1046-51. [PMID:22037378]
5. Drilon A, Ou SI, Cho BC, Kim DW, Lee J, Lin JJ, Zhu VW, Ahn MJ, Camidge DR, Nguyen J et al.. (2018) Repotrectinib (TPX-0005) Is a Next-Generation ROS1/TRK/ALK Inhibitor That Potently Inhibits ROS1/TRK/ALK Solvent- Front Mutations. Cancer Discov, 8 (10): 1227-1236. [PMID:30093503]
6. Iida H, Fujikawa R, Kozaki R, Harada R, Hosokawa Y, Ogawara KI, Ohno T. (2020) Pharmacokinetic-Pharmacodynamic-Efficacy Modeling of ONO-7579, a Novel Pan-Tropomyosin Receptor Kinase Inhibitor, in a Murine Xenograft Tumor Model. J Pharmacol Exp Ther, 373 (3): 361-369. [PMID:32217770]
7. Ito T, Kinoshita K, Tomizawa M, Shinohara S, Nishii H, Matsushita M, Hattori K, Kohchi Y, Kohchi M, Hayase T et al.. (2022) Discovery of CH7057288 as an Orally Bioavailable, Selective, and Potent pan-TRK Inhibitor. J Med Chem, 65 (18): 12427-12444. [PMID:36066182]
8. Kane JL Jr, Matthews G, Metz M, Kothe M, Liu J, Scholte A. (2015) Tropomyosin-related kinase (Trk) inhibitors. Patent number: US9174986B2. Assignee: Genzyme Corp.. Priority date: 10/12/2013. Publication date: 03/11/2015.
9. Katayama R, Gong B, Togashi N, Miyamoto M, Kiga M, Iwasaki S, Kamai Y, Tominaga Y, Takeda Y, Kagoshima Y et al.. (2019) The new-generation selective ROS1/NTRK inhibitor DS-6051b overcomes crizotinib resistant ROS1-G2032R mutation in preclinical models. Nat Commun, 10 (1): 3604. [PMID:31399568]
10. Li Y, Xiong Y, Zhang G, Zhang L, Yang W, Yang J, Huang L, Qiao Z, Miao Z, Lin G et al.. (2018) Identification of 5-(2,3-Dihydro-1 H-indol-5-yl)-7 H-pyrrolo[2,3- d]pyrimidin-4-amine Derivatives as a New Class of Receptor-Interacting Protein Kinase 1 (RIPK1) Inhibitors, Which Showed Potent Activity in a Tumor Metastasis Model. J Med Chem, 61 (24): 11398-11414. [PMID:30480444]
11. Li Z, Hu Y, Wei W, Liguang D, Xiaowei D, Yanqing Y, Yinghui S, Yongxin H, Yong P, Fansheng K. (2018) Amino pyrazolopyrimidine compound used as neurotrophic factor tyrosine kinase receptor inhibitor. Patent number: WO2018077246A1. Assignee: Chia Tai Tianqing Pharmaceutical Group Co., Ltd., Beijing Sailintai Pharmaceutical Technology Co., Ltd., Lianyungang Runzhong Pharmaceutical Co., Ltd.. Priority date: 27/10/2017. Publication date: 03/05/2018.
12. Nanda N, Bilenker JH, Doebele RC, Blake JF, Kolakowski GR, Brandhuber BJ, Andrews SW. (2017) Point mutations in trk inhibitor-resistant cancer and methods relating to the same. Patent number: WO2017075107A1. Assignee: Array Biopharma Inc, Loxo Oncology Inc. Priority date: 26/10/2015. Publication date: 04/05/2017.
13. Tap WD, Wainberg ZA, Anthony SP, Ibrahim PN, Zhang C, Healey JH, Chmielowski B, Staddon AP, Cohn AL, Shapiro GI et al.. (2015) Structure-Guided Blockade of CSF1R Kinase in Tenosynovial Giant-Cell Tumor. N Engl J Med, 373 (5): 428-37. [PMID:26222558]
14. Ultsch MH, Wiesmann C, Simmons LC, Henrich J, Yang M, Reilly D, Bass SH, de Vos AM. (1999) Crystal structures of the neurotrophin-binding domain of TrkA, TrkB and TrkC. J Mol Biol, 290 (1): 149-59. [PMID:10388563]
15. Wang Z, Zhang Y, Pinkas DM, Fox AE, Luo J, Huang H, Cui S, Xiang Q, Xu T, Xun Q et al.. (2018) Design, Synthesis, and Biological Evaluation of 3-(Imidazo[1,2- a]pyrazin-3-ylethynyl)-4-isopropyl- N-(3-((4-methylpiperazin-1-yl)methyl)-5-(trifluoromethyl)phenyl)benzamide as a Dual Inhibitor of Discoidin Domain Receptors 1 and 2. J Med Chem, 61 (17): 7977-7990. [PMID:30075624]
16. Wodicka LM, Ciceri P, Davis MI, Hunt JP, Floyd M, Salerno S, Hua XH, Ford JM, Armstrong RC, Zarrinkar PP et al.. (2010) Activation state-dependent binding of small molecule kinase inhibitors: structural insights from biochemistry. Chem Biol, 17 (11): 1241-9. [PMID:21095574]
17. Zhu Y, Ma Y, Zu W, Song J, Wang H, Zhong Y, Li H, Zhang Y, Gao Q, Kong B et al.. (2020) Identification of N-Phenyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine Derivatives as Novel, Potent, and Selective NF-κB Inducing Kinase (NIK) Inhibitors for the Treatment of Psoriasis. J Med Chem, 63 (13): 6748-6773. [PMID:32479083]
How to cite this page
Type VII RTKs: Neurotrophin receptor/Trk family: neurotrophic receptor tyrosine kinase 3. Last modified on 21/02/2023. Accessed on 19/04/2024. IUPHAR/BPS Guide to PHARMACOLOGY, https://www.guidetomalariapharmacology.org/GRAC/ObjectDisplayForward?objectId=1819.